
A spectrum is a condition that is not limited to a specific set of values but can vary, without gaps, across a continuum. The word spectrum was first used scientifically in optics to describe the rainbow of colors in visible light after passing through a prism. As scientific understanding of light advanced, it came to apply to the entire electromagnetic spectrum. It thereby became a mapping of a range of magnitudes (wavelengths) to a range of qualities, which are the perceived "colors of the rainbow" and other properties which correspond to wavelengths that lie outside of the visible light spectrum.

Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.

Idarubicin or 4-demethoxydaunorubicin is an anthracycline antileukemic drug. It inserts itself into DNA and prevents DNA unwinding by interfering with the enzyme topoisomerase II. It is an analog of daunorubicin, but the absence of a methoxy group increases its fat solubility and cellular uptake. Similar to other anthracyclines, it also induces histone eviction from chromatin.
An antimetabolite is a chemical that inhibits the use of a metabolite, which is another chemical that is part of normal metabolism. Such substances are often similar in structure to the metabolite that they interfere with, such as the antifolates that interfere with the use of folic acid; thus, competitive inhibition can occur, and the presence of antimetabolites can have toxic effects on cells, such as halting cell growth and cell division, so these compounds are used as chemotherapy for cancer.

Anthracyclines are a class of drugs used in cancer chemotherapy that are extracted from Streptomyces bacterium. These compounds are used to treat many cancers, including leukemias, lymphomas, breast, stomach, uterine, ovarian, bladder cancer, and lung cancers. The first anthracycline discovered was daunorubicin, which is produced naturally by Streptomyces peucetius, a species of Actinomycetota. Clinically the most important anthracyclines are doxorubicin, daunorubicin, epirubicin and idarubicin.

In mass spectrometry, matrix-assisted laser desorption/ionization (MALDI) is an ionization technique that uses a laser energy-absorbing matrix to create ions from large molecules with minimal fragmentation. It has been applied to the analysis of biomolecules and various organic molecules, which tend to be fragile and fragment when ionized by more conventional ionization methods. It is similar in character to electrospray ionization (ESI) in that both techniques are relatively soft ways of obtaining ions of large molecules in the gas phase, though MALDI typically produces far fewer multi-charged ions.

Stable Isotope Labeling by/with Amino acids in Cell culture (SILAC) is a technique based on mass spectrometry that detects differences in protein abundance among samples using non-radioactive isotopic labeling. It is a popular method for quantitative proteomics.
Topoisomerase inhibitors are chemical compounds that block the action of topoisomerases, which are broken into two broad subtypes: type I topoisomerases (TopI) and type II topoisomerases (TopII). Topoisomerase plays important roles in cellular reproduction and DNA organization, as they mediate the cleavage of single and double stranded DNA to relax supercoils, untangle catenanes, and condense chromosomes in eukaryotic cells. Topoisomerase inhibitors influence these essential cellular processes. Some topoisomerase inhibitors prevent topoisomerases from performing DNA strand breaks while others, deemed topoisomerase poisons, associate with topoisomerase-DNA complexes and prevent the re-ligation step of the topoisomerase mechanism. These topoisomerase-DNA-inhibitor complexes are cytotoxic agents, as the un-repaired single- and double stranded DNA breaks they cause can lead to apoptosis and cell death. Because of this ability to induce apoptosis, topoisomerase inhibitors have gained interest as therapeutics against infectious and cancerous cells.
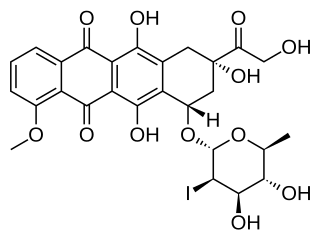
Annamycin is an anthracycline antibiotic being investigated for the treatment of cancer.

Pirarubicin (INN) is an anthracycline drug. An analogue of the anthracycline antineoplastic antibiotic doxorubicin. Pirarubicin intercalates into DNA and interacts with topoisomerase II, thereby inhibiting DNA replication and repair and RNA and protein synthesis. This agent is less cardiotoxic than doxorubicin and exhibits activity against some doxorubicin-resistant cell lines.

Nogalamycin is an anthracycline antibiotic produced by the soil bacteria Streptomyces nogalater. It has antitumor properties but it is also highly cardiotoxic. The less cardiotoxic semisynthetic analog menogaril was developed in the 1970s. Currently nogalamycin and menogaril are not used clinically.
Bioanalysis is a sub-discipline of analytical chemistry covering the quantitative measurement of xenobiotics and biotics in biological systems.
Peptaibols are biologically active peptides containing between seven and twenty amino acid residues, some of which are non-proteinogenic amino acids. In particular, they contain α-aminoisobutyric acid along with other unusual aminoacids such as ethylnorvaline, isovaline and hydroxyproline; the N-terminus is acetylated, and the C-terminal amino acid is hydroxylated to an acid alcohol. They are named pebtaibols due to them being peptides containing α-aminoisobutyric acid (Aib) and ending in an alcohol. They are produced by certain fungi, mainly in the genus Trichoderma, as secondary metabolites which function as antibiotics and antifungal agents. Some are referred to as trichorzianines. They are amphipathic which allows them to form voltage-dependent ion channels in cell membranes which create holes in the membrane making them leaky and leading to the death of the cells. As of 2001, over 317 peptaibols had been identified. The most widely known peptaibol is alamethicin.
Bohemic acid is a mixture of chemical compounds which is obtained through fermentation by actinobacteria species in the genus Actinosporangium (Actinoplanaceae). The name honors the Puccini opera La Bohème and many individual components of the acid carry the names of characters from La Bohème. Most of those components are antitumor agents and anthracycline antibiotics active against Gram-positive bacteria.

Collision-induced dissociation (CID), also known as collisionally activated dissociation (CAD), is a mass spectrometry technique to induce fragmentation of selected ions in the gas phase. The selected ions are usually accelerated by applying an electrical potential to increase the ion kinetic energy and then allowed to collide with neutral molecules. In the collision, some of the kinetic energy is converted into internal energy which results in bond breakage and the fragmentation of the molecular ion into smaller fragments. These fragment ions can then be analyzed by tandem mass spectrometry.
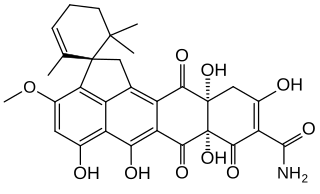
Viridicatumtoxin B is a fungus-derived tetracycline-like antibiotic discovered in 2008. It was isolated from small amounts of penicillium fungi. A synthetic structure matching that of natural viridicatumtoxin B makes possible synthetic variants that match or surpass its antibiotic potency.
Streptomyces capoamus is a bacterium species from the genus of Streptomyces which has been isolated from soil from Iceland. Streptomyces capoamus produces capomycin, ciclamycin O, ciclamycin 4, anthracycline, ciclacidin A, ciclacidin B and ciclamicin.
Streptomyces violaceus is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces violaceus produces rhodomycine, violamycin-B5 and violarin B.

Dudley Howard Williams (1937–2010) was a British biochemist known for utilizing nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry in the study of molecular structure, especially the antibiotic vancomycin.

Tetracenomycin C is an antitumor anthracycline-like antibiotic produced by Streptomyces glaucescens GLA.0. The pale-yellow antibiotic is active against some gram-positive bacteria, especially against streptomycetes. Gram-negative bacteria and fungi are not inhibited. In considering the differences of biological activity and the functional groups of the molecule, tetracenomycin C is not a member of the tetracycline or anthracyclinone group of antibiotics. Tetracenomycin C is notable for its broad activity against actinomycetes. As in other anthracycline antibiotics, the framework is synthesized by a polyketide synthase and subsequently modified by other enzymes.













