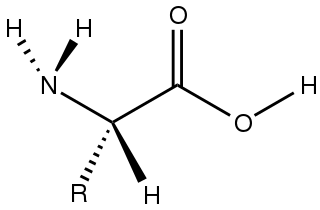
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code.

Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐CH2‐COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a Clostridium tetani infection) can cause spastic paralysis due to uninhibited muscle contraction.

The genetic code is the set of rules used by living cells to translate information encoded within genetic material into proteins. Translation is accomplished by the ribosome, which links proteinogenic amino acids in an order specified by messenger RNA (mRNA), using transfer RNA (tRNA) molecules to carry amino acids and to read the mRNA three nucleotides at a time. The genetic code is highly similar among all organisms and can be expressed in a simple table with 64 entries.
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group -NH
2 but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG).
An essential amino acid, or indispensable amino acid, is an amino acid that cannot be synthesized from scratch by the organism fast enough to supply its demand, and must therefore come from the diet. Of the 21 amino acids common to all life forms, the nine amino acids humans cannot synthesize are phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, and histidine.

Bumetanide, sold under the brand name Bumex among others, is a medication used to treat swelling and high blood pressure. This includes swelling as a result of heart failure, liver failure, or kidney problems. It may work for swelling when other medications have not. For high blood pressure it is not a preferred treatment. It is taken by mouth, or by injection into a vein or muscle. Effects generally begin within an hour and lasts for about six hours.

The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."

The Weinreb–Nahm ketone synthesis is a chemical reaction used in organic chemistry to make carbon–carbon bonds. It was discovered in 1981 by Steven M. Weinreb and Steven Nahm as a method to synthesize ketones. The original reaction involved two subsequent nucleophilic acyl substitutions: the conversion of an acid chloride with N,O-Dimethylhydroxylamine, to form a Weinreb–Nahm amide, and subsequent treatment of this species with an organometallic reagent such as a Grignard reagent or organolithium reagent. Nahm and Weinreb also reported the synthesis of aldehydes by reduction of the amide with an excess of lithium aluminum hydride.
Lipotropin is the name for two hormones produced by the cleavage of pro-opiomelanocortin (POMC). The anterior pituitary gland produces the pro-hormone POMC, which is then cleaved again to form adrenocorticotropin (ACTH) and β-lipotropin (β-LPH).
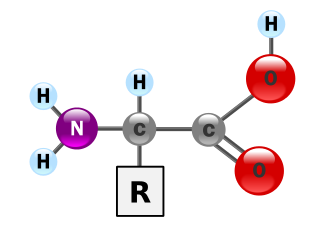
Proteins are essential nutrients for the human body. They are one of the building blocks of body tissue and can also serve as a fuel source. As a fuel, proteins provide as much energy density as carbohydrates: 4 kcal per gram; in contrast, lipids provide 9 kcal per gram. The most important aspect and defining characteristic of protein from a nutritional standpoint is its amino acid composition.
In chemical nomenclature, nor- is a prefix to name a structural analog that can be derived from a parent compound by the removal of one carbon atom along with the accompanying hydrogen atoms. The nor-compound can be derived by removal of a CH
3, CH
2, or CH group, or of a C atom. The "nor-" prefix also includes the elimination of a methylene bridge in a cyclic parent compound, followed by ring contraction.. The terms desmethyl- or demethyl- are synonyms of "nor-".
Neurokinin 1 (NK1) antagonists (-pitants) are a novel class of medications that possesses unique antidepressant, anxiolytic, and antiemetic properties. NK-1 antagonists boost the efficacy of 5-HT3 antagonists to prevent nausea and vomiting. The discovery of neurokinin 1 (NK1) receptor antagonists was a turning point in the prevention of nausea and vomiting associated with cancer chemotherapy.
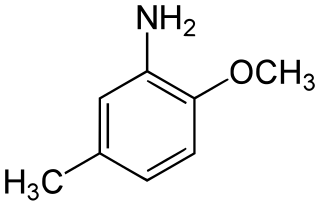
para-Cresidine is an organic compound with the formula CH3OC6H3(CH3)NH2. It is a white solid that is soluble in organic solvents. The compound features both amine and methoxy functional groups. It is used as an intermediate in preparation of dyes and pigments.

Neutral amino acid transporter B(0) is a protein that in humans is encoded by the SLC1A5 gene.

Neutral amino acid transporter A is a protein that in humans is encoded by the SLC1A4 gene.

Methoxy polyethylene glycol-epoetin beta is the active ingredient of a drug marketed by Hoffmann-La Roche under the brand name Mircera. Mircera is a long-acting erythropoietin receptor activator (CERA) indicated for the treatment of patients with anaemia associated with chronic kidney disease. It is the first approved, chemically modified erythropoiesis-stimulating agent (ESA). Mircera is supplied as a solution in pre-filled syringes for intravenous or subcutaneous administration. Mircera was approved for use in Europe in July 2007 by the European Commission, in September 2007 by the Swissmedic, and in November 2007 by the U.S. Food and Drug Administration for use in the United States.

Melatonin receptor agonists are analogues of melatonin that bind to and activate the melatonin receptor. Agonists of the melatonin receptor have a number of therapeutic applications including treatment of sleep disorders and depression. The discovery and development of melatonin receptor agonists was motivated by the need for more potent analogues than melatonin, with better pharmacokinetics and longer half-lives. Melatonin receptor agonists were developed with the melatonin structure as a model.
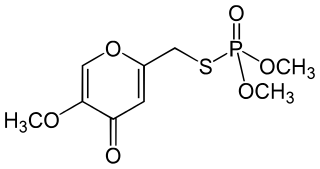
Endothion is an organic compound used as an insecticide and acaricides. It is part of the chemical class of organophosphorus compounds. It is generally described as white crystals with a slight odor. It is used as an insecticide, but not sold in the United States or Canada.
ADDA is a non-proteinogenic amino acid found in toxins made by cyanobacteria. Toxins which include this amino acid include microcystins and nodularins.

Mexedrone is a stimulant and an entactogen drug of the cathinone class that has been sold online as a designer drug. It is the alpha-methoxy derivative of Mephedrone.














