
Venom or zootoxin is a type of toxin produced by an animal that is actively delivered through a wound by means of a bite, sting, or similar action. The toxin is delivered through a specially evolved venom apparatus, such as fangs or a stinger, in a process called envenomation. Venom is often distinguished from poison, which is a toxin that is passively delivered by being ingested, inhaled, or absorbed through the skin, and toxungen, which is actively transferred to the external surface of another animal via a physical delivery mechanism.

Phoneutria is a genus of spiders in the family Ctenidae. They are mainly found in northern South America, with one species in Central America. Members of the genus are commonly referred to as Brazilian wandering spiders. Other English names include armed spiders and banana spiders.

Latrodectus is a broadly distributed genus of spiders with several species that are commonly known as the true widows. This group is composed of those often loosely called black widow spiders, brown widow spiders, and similar spiders. However, the diversity of species is much greater. A member of the family Theridiidae, this genus contains 34 species, which include several North American "black widows". Besides these, North America also has the red widow Latrodectus bishopi and the brown widow Latrodectus geometricus, which, in addition to North America, has a much wider geographic distribution. Elsewhere, others include the European black widow, the Australian redback spider and the closely related New Zealand katipō, several different species in Southern Africa that can be called button spiders, and the South American black-widow spiders. Species vary widely in size. In most cases, the females are dark-coloured and can be readily identified by reddish markings on the central underside (ventral) abdomen, which are often hourglass-shaped.

The Chinese red-headed centipede, also known as the Chinese red head, is a centipede from East Asia. It averages 20 cm (8 in) in length and lives in damp environments.
A latrotoxin is a high-molecular mass neurotoxin found in the venom of spiders of the genus Latrodectus as well as at least one species of another genus in the same family, Steatoda nobilis. Latrotoxins are the main active components of the venom and are responsible for the symptoms of latrodectism.

A spider bite, also known as arachnidism, is an injury resulting from the bite of a spider. The effects of most bites are not serious. Most bites result in mild symptoms around the area of the bite. Rarely they may produce a necrotic skin wound or severe pain.

Latrodectus hesperus, the western black widow spider or western widow, is a venomous spider species found in western regions of North America. The female's body is 14–16 mm in length and is black, often with an hourglass-shaped red mark on the lower abdomen. This "hourglass" mark can be yellow, and on rare occasions, white. The male of the species is around half this length and generally a tan color with lighter striping on the abdomen. The population was previously described as a subspecies of Latrodectus mactans and it is closely related to the northern species Latrodectus variolus. The species, as with others of the genus, build irregular or "messy" webs: unlike the spiral webs or the tunnel-shaped webs of other spiders, the strands of a Latrodectus web have no apparent organization.

Phoneutria nigriventer is a species of medically significant spider in the family Ctenidae, found in the Southern Cone of South America. Along with other members of the genus, they are often referred to as Brazilian wandering spiders.
Tx2-6 is a toxin found in the venom of the Brazilian wandering spider, Phoneutria nigriventer(Keyserling). It is a peptide of 48 residues, molecular weight 5291.3. This peptide is cleaved from a longer precursor with a signal peptide and a glutamine-rich propeptide. It can cause priapism. Tests on rats indicate that the toxin causes nitric oxide release, and its effect on erection is blocked by the nitric oxide synthase inhibitor L-NAME. However, it fully restored erectile function in rats developing hypertension due to injection of deoxycorticosterone acetate. A study is underway at the Medical College of Georgia looking at possible uses for the chemical in erectile dysfunction medication. Scientists and Gregory Ochs are collaborating on this study.
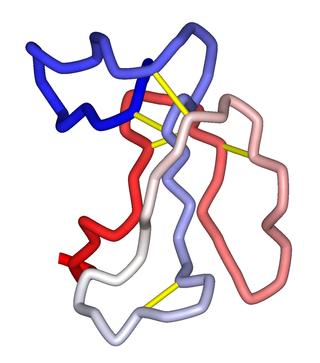
α-Neurotoxins are a group of neurotoxic peptides found in the venom of snakes in the families Elapidae and Hydrophiidae. They can cause paralysis, respiratory failure, and death. Members of the three-finger toxin protein family, they are antagonists of post-synaptic nicotinic acetylcholine receptors (nAChRs) in the neuromuscular synapse that bind competitively and irreversibly, preventing synaptic acetylcholine (ACh) from opening the ion channel. Over 100 α-neurotoxins have been identified and sequenced.
Venom optimization hypothesis, also known as venom metering, is a biological hypothesis which postulates that venomous animals have physiological control over their production and use of venoms. It explains the economic use of venom because venom is a metabolically expensive product, and that there is a biological mechanism for controlling their specific use. The hypothetical concept was proposed by Esther Wigger, Lucia Kuhn-Nentwig, and Wolfgang Nentwig of the Zoological Institute at the University of Bern, Switzerland, in 2002.
CSTX is a name given to a group of closely related neurotoxic peptides present in the venom of the wandering spider Cupiennius salei. There are twenty types so far described for this protein group. However, some are reclassified into cupiennins group of toxin, including CSTX-3, -4, -5, and -6, because of their chemical affinity. The first thirteen were isolated and identified in 1994 by Lucia Kuhn-Nentwig, Johann Schaller, and Wolfgang Nentwig of the Zoological Institute at the University of Bern, Switzerland. The different types are most likely the products of splicing variant of the same gene. They are all L-type calcium channel blockers, and also exhibit cytolytic activity by forming an alpha-helix across the cell membrane in mammalian neurons. They also inhibit voltage-gated calcium channels in insect neurons.
Cupiennins are a group of small cytolytic peptides from the venom of the wandering spider Cupiennius salei. They are known to have high bactericidal, insecticidal and haemolytic activities. They are chemically cationic α-helical peptides. They were isolated and identified in 2002 as a family of peptides called cupiennin 1. The sequence was determined by a process called Edman degradation, and the family consists of cupiennin 1a, cupiennin 1b, cupiennin 1c, and cupiennin 1d. The amino acid sequences of cupiennin 1b, c, and d were obtained by a combination of sequence analysis and mass spectrometric measurements of comparative tryptic peptide mapping. Even though they are not strong toxins, they do enhance the effect of the spider venom by synergistically enhancing other components of the venom, such CSTX.
Oxotoxins, or oxytoxins, are a group of neurotoxins present in the venom of lynx spiders belonging to the genus Oxyopes, hence the name oxytoxin. They are disulfide-rich peptides. Only two types are so far reported from two different species, the larger oxytoxin 1 (OxyTx1) from Oxyopes kitabensis, and the smaller oxytoxin 2 (OxyTx2) from Oxyopes lineatus. OxyTx1, the first known oxytoxin, was discovered in 2002. It was found to enhance the lethal efficacy of the spider venom by acting together with oxyopinins. It is composed of 69 amino acid residue, which are cross-linked by five disulfide bridges. It is a large peptide having a molecular mass of 8059.2 Da; but shows the size of 9,109.4 Da due to the presence of disulfide bridges. It is a potent insecticide, but non-toxic to mice up to 1 μg/20-g mouse. It acts synergistically with oxyopinins of the same venom to increase the insecticidal effect.
Apomastus schlingeri is a species of venomous spiders belonging to a family of trapdoor spiders. They produce a complex of neurotoxins called aptotoxins. Both known species of the genus are found in the United States.

Dolomedes sulfureus is a species of spiders commonly known as fishing spiders belonging to the genus Dolomedes. They produce a venom that contains a group of neurotoxic peptides. The species is found in Russia, China, Korea, and Japan.
The pathophysiology of a spider bite is due to the effect of its venom. A spider envenomation occurs whenever a spider injects venom into the skin. Not all spider bites inject venom – a dry bite, and the amount of venom injected can vary based on the type of spider and the circumstances of the encounter. The mechanical injury from a spider bite is not a serious concern for humans. Some spider bites do leave a large enough wound that infection may be a concern. However, it is generally the toxicity of spider venom that poses the most risk to human beings; several spiders are known to have venom that can cause injury to humans in the amounts that a spider will typically inject when biting.
Venomics is the large-scale study of proteins associated with venom. Venom is a toxic substance secreted by animals, which is typically injected either offensively or defensively into prey or aggressors, respectively.
U7-ctenitoxin-Pn1a (or U7-CNTX-Pn1a for short) is a neurotoxin that blocks TRPV1 channels, and can exhibit analgestic effects. It is naturally found in the venom of Phoneutria nigriventer.

Delta hexatoxin Hv1 is a neurotoxic component found in the venom of the Australian funnel web spider.














