Related Research Articles
Phycobilins are light-capturing bilins found in cyanobacteria and in the chloroplasts of red algae, glaucophytes and some cryptomonads. Most of their molecules consist of a chromophore which makes them coloured. They are unique among the photosynthetic pigments in that they are bonded to certain water-soluble proteins, known as phycobiliproteins. Phycobiliproteins then pass the light energy to chlorophylls for photosynthesis.

Phytochromes are a class of photoreceptor in plants, bacteria and fungi used to detect light. They are sensitive to light in the red and far-red region of the visible spectrum and can be classed as either Type I, which are activated by far-red light, or Type II that are activated by red light. Recent advances have suggested that phytochromes also act as temperature sensors, as warmer temperatures enhance their de-activation. All of these factors contribute to the plant's ability to germinate.
In developmental biology, photomorphogenesis is light-mediated development, where plant growth patterns respond to the light spectrum. This is a completely separate process from photosynthesis where light is used as a source of energy. Phytochromes, cryptochromes, and phototropins are photochromic sensory receptors that restrict the photomorphogenic effect of light to the UV-A, UV-B, blue, and red portions of the electromagnetic spectrum.
Far-red light is a range of light at the extreme red end of the visible spectrum, just before infra-red light. Usually regarded as the region between 700 and 750 nm wavelength, it is dimly visible to human eyes. It is largely reflected or transmitted by plants because of the absorbance spectrum of chlorophyll, and it is perceived by the plant photoreceptor phytochrome. However, some organisms can use it as a source of energy in photosynthesis. Far-red light also is used for vision by certain organisms such as some species of deep-sea fishes and mantis shrimp.
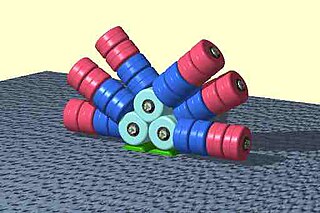
Phycobilisomes are light harvesting antennae of photosystem II in cyanobacteria, red algae and glaucophytes. It was lost in the plastids of green algae/ plants (chloroplasts).
Photoprotection is the biochemical process that helps organisms cope with molecular damage caused by sunlight. Plants and other oxygenic phototrophs have developed a suite of photoprotective mechanisms to prevent photoinhibition and oxidative stress caused by excess or fluctuating light conditions. Humans and other animals have also developed photoprotective mechanisms to avoid UV photodamage to the skin, prevent DNA damage, and minimize the downstream effects of oxidative stress.

Biological pigments, also known simply as pigments or biochromes, are substances produced by living organisms that have a color resulting from selective color absorption. Biological pigments include plant pigments and flower pigments. Many biological structures, such as skin, eyes, feathers, fur and hair contain pigments such as melanin in specialized cells called chromatophores. In some species, pigments accrue over very long periods during an individual's lifespan.

Bilins, bilanes or bile pigments are biological pigments formed in many organisms as a metabolic product of certain porphyrins. Bilin was named as a bile pigment of mammals, but can also be found in lower vertebrates, invertebrates, as well as red algae, green plants and cyanobacteria. Bilins can range in color from red, orange, yellow or brown to blue or green.
Photoreceptor proteins are light-sensitive proteins involved in the sensing and response to light in a variety of organisms. Some examples are rhodopsin in the photoreceptor cells of the vertebrate retina, phytochrome in plants, and bacteriorhodopsin and bacteriophytochromes in some bacteria. They mediate light responses as varied as visual perception, phototropism and phototaxis, as well as responses to light-dark cycles such as circadian rhythm and other photoperiodisms including control of flowering times in plants and mating seasons in animals.
Synechocystis sp. PCC6803 is a strain of unicellular, freshwater cyanobacteria. Synechocystis sp. PCC6803 is capable of both phototrophic growth by oxygenic photosynthesis during light periods and heterotrophic growth by glycolysis and oxidative phosphorylation during dark periods. Gene expression is regulated by a circadian clock and the organism can effectively anticipate transitions between the light and dark phases.

Phototaxis is a kind of taxis, or locomotory movement, that occurs when a whole organism moves towards or away from a stimulus of light. This is advantageous for phototrophic organisms as they can orient themselves most efficiently to receive light for photosynthesis. Phototaxis is called positive if the movement is in the direction of increasing light intensity and negative if the direction is opposite.

The psaA RNA motif describes a class of RNAs with a common secondary structure. psaA RNAs are exclusively found in locations that presumably correspond to the 5' untranslated regions of operons formed of psaA and psaB genes. For this reason, it was hypothesized that psaA RNAs function as cis-regulatory elements of these genes. The psaAB genes encode proteins that form subunits in the photosystem I structure used for photosynthesis. psaA RNAs have been detected only in cyanobacteria, which is consistent with their association with photosynthesis.
In molecular biology, the BtpA protein family is a family of proteins which includes BtpA. BtpA appears to play a role in the stabilisation of photosystem I. It is an extrinsic membrane protein located on the cytoplasmic side of the thylakoid membrane. Homologs of BtpA are found in the crenarchaeota and euryarchaeota, where their function remains unknown. The Ycf4 protein is firmly associated with the thylakoid membrane, presumably through a transmembrane domain. Ycf4 co-fractionates with a protein complex larger than PSI upon sucrose density gradient centrifugation of solubilised thylakoids. The Ycf3 protein is loosely associated with the thylakoid membrane and can be released from the membrane with sodium carbonate. This suggests that Ycf3 is not part of a stable complex and that it probably interacts transiently with its partners. Ycf3 contains a number of tetratricopeptide repeats (TPR); TPR is a structural motif present in a wide range of proteins, which mediates protein-protein interactions.
Plastoglobulins is a family of proteins prominent found in lipid globules in plastids of flowering plants. It shows sequence similarities to the PAP/fibrillin family. PGL and similar proteins can be found in most algae, cyanobacteria and plants, but no other life forms; it suggests a role for PGL in oxygenic photosynthesis.
In molecular biology, Cyanobacterial non-coding RNAs are non-coding RNAs which have been identified in species of cyanobacteria. Large scale screens have identified 21 Yfr in the marine cyanobacterium Prochlorococcus and related species such as Synechococcus. These include the Yfr1 and Yfr2 RNAs. In Prochlorococcus and Synechocystis, non-coding RNAs have been shown to regulate gene expression. NsiR4, widely conserved throughout the cyanobacterial phylum, has been shown to be involved in nitrogen assimilation control in Synechocystis sp. PCC 6803 and in the filamentous, nitrogen-fixing Anabaena sp. PCC 7120.
kaiA is a gene in the "kaiABC" gene cluster that plays a crucial role in the regulation of bacterial circadian rhythms, such as in the cyanobacterium Synechococcus elongatus. For these bacteria, regulation of kaiA expression is critical for circadian rhythm, which determines the twenty-four-hour biological rhythm. In addition, KaiA functions with a negative feedback loop in relation with kaiB and KaiC. The kaiA gene makes KaiA protein that enhances phosphorylation of KaiC while KaiB inhibits activity of KaiA.
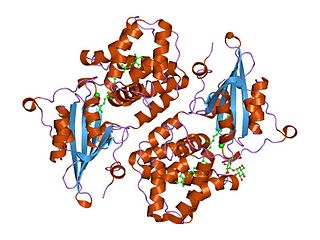
Orange carotenoid protein (OCP) is a water-soluble protein which plays a role in photoprotection in diverse cyanobacteria. It is the only photoactive protein known to use a carotenoid as the photoresponsive chromophore. The protein consists of two domains, with a single keto-carotenoid molecule non-covalently bound between the two domains. It is a very efficient quencher of excitation energy absorbed by the primary light-harvesting antenna complexes of cyanobacteria, the phycobilisomes. The quenching is induced by blue-green light. It is also capable of preventing oxidative damage by directly scavenging singlet oxygen (1O2).
Fluorescence recovery protein (FRP) is a small protein involved in regulating non-photochemical quenching in cyanobacteria. It prevents accumulation of the red photoactivated form of orange carotenoid protein (OCP), thereby reducing the amount of fluorescence quenching that occurs between the OCP and the phycobilisome antenna complexes. It interacts with the C-terminal domain of OCP, which shares homology with the NTF2 superfamily.
Myxoxanthophyll is a carotenoid glycoside pigment present in the photosynthetic apparatus of cyanobacteria. It is named after the word "Myxophyceae", a former term for cyanobacteria. As a monocyclic xanthophyll, it has a yellowish color. It is required for normal cell wall structure and thylakoid organization in the cyanobacterium Synechocystis. The pigment is unusual because it is glycosylated on the 2'-OH rather than the 1'-OH position of the molecule. Myxoxanthophyll was first isolated from Oscillatoria rubenscens in 1936.

Ethylene signaling pathway is a signal transduction in plant cells to regulate important growth and developmental processes. Acting as a plant hormone, the gas ethylene is responsible for promoting the germination of seeds, ripening of fruits, the opening of flowers, the abscission of leaves and stress responses. It is the simplest alkene gas and the first gaseous molecule discovered to function as a hormone.
References
- ↑ Ikeuchi, M; T Ishizuka (2008-10-07). "Cyanobacteriochromes: a new superfamily of tetrapyrrole-binding photoreceptors in cyanobacteria". Photochemical & Photobiological Sciences. RSC Publishing. 7 (10): 1159–67. doi:10.1039/b802660m.
- ↑ Rockwell, Nathan C.; Njuguna, Stephanie Lane; Roberts, Laurel; Castillo, Elenor; Parson, Victoria L.; Dwojak, Sunshine; Lagarias, J. Clark; Spiller, Susan C. (2008-07-08). "A second conserved GAF domain cysteine is required for the blue/green photoreversibility of cyanobacteriochrome Tlr0924 from Thermosynechococcus elongatus". Biochemistry. 47 (27): 7304–7316. doi:10.1021/bi800088t. ISSN 0006-2960. PMC 2574597 . PMID 18549244.