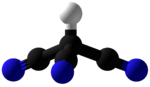
Cyanogen is the chemical compound with the formula (CN)2. The simplest stable carbon nitride, it is a colorless and highly toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups ‒ analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms: N≡C‒C≡N, though other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide (NCBr) (but see also Cyano radical). When burned at increased pressure with oxygen, it is possible to get a blue tinted flame, the temperature of which is about 4800°C (a higher temperature is possible with ozone). It is as such regarded as the gas with the second highest temperature of burning (after dicyanoacetylene).

In organic chemistry, a cyanohydrin or hydroxynitrile is a functional group found in organic compounds in which a cyano and a hydroxy group are attached to the same carbon atom. The general formula is R2C(OH)CN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids. Cyanohydrins can be formed by the cyanohydrin reaction, which involves treating a ketone or an aldehyde with hydrogen cyanide (HCN) in the presence of excess amounts of sodium cyanide (NaCN) as a catalyst:
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The name of the compound is composed of a base, which includes the carbon of the −C≡N, suffixed with "nitrile", so for example CH3CH2C≡N is called "propionitrile". The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
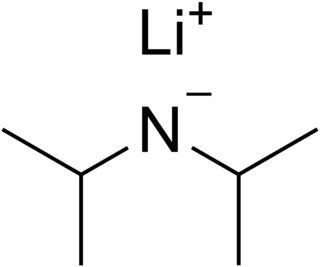
Lithium diisopropylamide is a chemical compound with the molecular formula LiN(CH 2)2. It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature. It is a colorless solid, but is usually generated and observed only in solution. It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group.
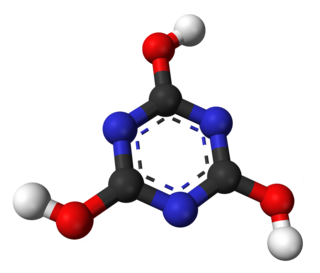
Cyanuric acid or 1,3,5-triazine-2,4,6-triol is a chemical compound with the formula (CNOH)3. Like many industrially useful chemicals, this triazine has many synonyms. This white, odorless solid finds use as a precursor or a component of bleaches, disinfectants, and herbicides. In 1997, worldwide production was 160 000 tonnes.
In organic chemistry, hydrocyanation is a process for conversion of alkenes to nitriles. The reaction involves the addition of hydrogen cyanide and requires a catalyst. This conversion is conducted on an industrial scale for the production of precursors to nylon.

Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, sulphamic acid and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colourless, water-soluble compound finds many applications. Sulfamic acid melts at 205 °C before decomposing at higher temperatures to water, sulfur trioxide, sulfur dioxide and nitrogen.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.
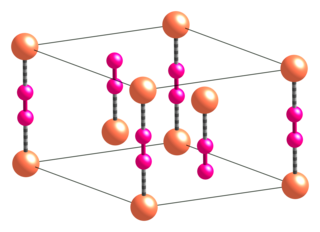
Copper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in electroplating copper, and as a reagent in the preparation of nitriles.

A persistent carbene is an organic molecule whose natural resonance structure has a carbon atom with incomplete octet, but does not exhibit the tremendous instability typically associated with such moieties. The best-known examples and by far largest subgroup are the N-heterocyclic carbenes (NHC), in which nitrogen atoms flank the formal carbene.
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula H2NCOOH. It can be obtained by the reaction of ammonia NH3 and carbon dioxide CO2 at very low temperatures, which also yields ammonium carbamate [NH4]+[NH2CO2]−. The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.
The Bucherer–Bergs reaction is the chemical reaction of carbonyl compounds or cyanohydrins with ammonium carbonate and potassium cyanide to give hydantoins. The reaction is named after Hans Theodor Bucherer.
The Chichibabin reaction is a method for producing 2-aminopyridine derivatives by the reaction of pyridine with sodium amide. It was reported by Aleksei Chichibabin in 1914. The following is the overall form of the general reaction:
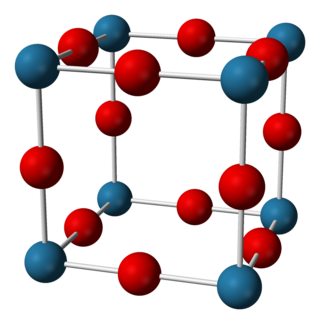
Sodium nitride is the inorganic compound with the chemical formula Na3N. In contrast to lithium nitride and some other nitrides, sodium nitride is an extremely unstable alkali metal nitride. It can be generated by combining atomic beams of sodium and nitrogen deposited onto a low-temperature sapphire substrate. It readily decomposes into its elements:

Methyltrichlorosilane, also known as trichloromethylsilane, is a monomer and organosilicon compound with the formula CH3SiCl3. It is a colorless liquid with a sharp odor similar to that of hydrochloric acid. As methyltrichlorosilane is a reactive compound, it is mainly used a precursor for forming various cross-linked siloxane polymers.

Tetramethylurea is the organic compound with the formula (Me2N)2CO. It is a substituted urea. This colorless liquid is used as an aprotic-polar solvent, especially for aromatic compounds and is used e. g. for Grignard reagents.

N-Hydroxyphthalimide is the organic compound with the formula C6H4(CO)2NOH. A white or yellow solid, it is a derivative of phthalimide. The compound is as a catalyst in the synthesis of other organic compounds. It is soluble in water and organic solvents such as acetic acid, ethyl acetate and acetonitrile.
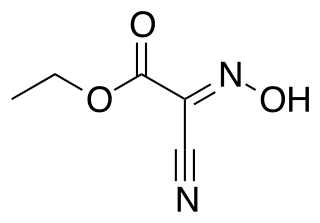
Ethyl cyanohydroxyiminoacetate (oxyma) is the oxime of ethyl cyanoacetate and finds use as an additive for carbodiimides, such as dicyclohexylcarbodiimide (DCC) in peptide synthesis. It acts as a neutralizing reagent for the basicity or nucleophilicity of the DCC due to its pronounced acidity and suppresses base catalyzed side reactions, in particular racemization.

Nitrilotriacetonitrile (NTAN) is a precursor for nitrilotriacetic acid, for tris(2-aminoethyl)amine and for the epoxy resin crosslinker aminoethylpiperazine.
1,4-butane sultone is a six-membered δ-sultone and the cyclic ester of 4-hydroxybutanesulfonic acid. As a sulfo-alkylating agent, 1,4-butanesultone is used to introduce the sulfobutyl group (–(CH2)4–SO3−) into hydrophobic compounds possessing nucleophilic functional groups, for example hydroxy groups (as in the case of β-cyclodextrin) or amino groups (as in the case of polymethine dyes). In such, the sulfobutyl group is present as neutral sodium salt and considerably increases the water solubility of the derivatives.