The term carotene (also carotin, from the Latin carota, "carrot") is used for many related unsaturated hydrocarbon substances having the formula C40Hx, which are synthesized by plants but in general cannot be made by animals (with the exception of some aphids and spider mites which acquired the synthesizing genes from fungi). Carotenes are photosynthetic pigments important for photosynthesis. Carotenes contain no oxygen atoms. They absorb ultraviolet, violet, and blue light and scatter orange or red light, and (in low concentrations) yellow light.

Lycopene is an organic compound classified as a tetraterpene and a carotene. Lycopene is a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and vegetables.

Carotenoids are yellow, orange, and red organic pigments that are produced by plants and algae, as well as several bacteria, archaea, and fungi. Carotenoids give the characteristic color to pumpkins, carrots, parsnips, corn, tomatoes, canaries, flamingos, salmon, lobster, shrimp, and daffodils. Over 1,100 identified carotenoids can be further categorized into two classes – xanthophylls and carotenes.

Phytochemicals are chemical compounds produced by plants, generally to help them resist fungi, bacteria and plant virus infections, and also consumption by insects and other animals. The name comes from Greek φυτόν (phyton) 'plant'. Some phytochemicals have been used as poisons and others as traditional medicine.

Pyruvate kinase is the enzyme involved in the last step of glycolysis. It catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP), yielding one molecule of pyruvate and one molecule of ATP. Pyruvate kinase was inappropriately named before it was recognized that it did not directly catalyze phosphorylation of pyruvate, which does not occur under physiological conditions. Pyruvate kinase is present in four distinct, tissue-specific isozymes in animals, each consisting of particular kinetic properties necessary to accommodate the variations in metabolic requirements of diverse tissues.

Methylcholanthrene is a highly carcinogenic polycyclic aromatic hydrocarbon produced by burning organic compounds at very high temperatures. Methylcholanthrene is also known as 3-methylcholanthrene, 20-methylcholanthrene or the IUPAC name 3-methyl-1,2-dyhydrobenzo[j]aceanthrylene. The short notation often used is 3-MC or MCA. This compound forms pale yellow solid crystals when crystallized from benzene and ether. It has a melting point around 180 °C and its boiling point is around 280 °C at a pressure of 80 mmHg. Methylcholanthrene is used in laboratory studies of chemical carcinogenesis. It is an alkylated derivative of benz[a]anthracene and has a similar UV spectrum. The most common isomer is 3-methylcholanthrene, although the methyl group can occur in other places.
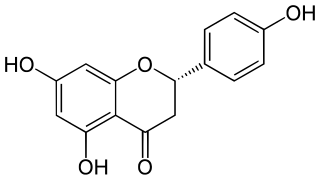
Naringenin is a flavorless, colorless flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit, and is found in a variety of fruits and herbs.

Gingerol ([6]-gingerol) is a phenolic phytochemical compound found in fresh ginger that activates heat receptors on the tongue. It is normally found as a pungent yellow oil in the ginger rhizome, but can also form a low-melting crystalline solid. This chemical compound is found in all members of the Zingiberaceae family and is high in concentrations in the grains of paradise as well as an African Ginger species.

Phosphofructokinase-2 (6-phosphofructo-2-kinase, PFK-2) or fructose bisphosphatase-2 (FBPase-2), is an enzyme indirectly responsible for regulating the rates of glycolysis and gluconeogenesis in cells. It catalyzes formation and degradation of a significant allosteric regulator, fructose-2,6-bisphosphate (Fru-2,6-P2) from substrate fructose-6-phosphate. Fru-2,6-P2 contributes to the rate-determining step of glycolysis as it activates enzyme phosphofructokinase 1 in the glycolysis pathway, and inhibits fructose-1,6-bisphosphatase 1 in gluconeogenesis. Since Fru-2,6-P2 differentially regulates glycolysis and gluconeogenesis, it can act as a key signal to switch between the opposing pathways. Because PFK-2 produces Fru-2,6-P2 in response to hormonal signaling, metabolism can be more sensitively and efficiently controlled to align with the organism's glycolytic needs. This enzyme participates in fructose and mannose metabolism. The enzyme is important in the regulation of hepatic carbohydrate metabolism and is found in greatest quantities in the liver, kidney and heart. In mammals, several genes often encode different isoforms, each of which differs in its tissue distribution and enzymatic activity. The family described here bears a resemblance to the ATP-driven phospho-fructokinases, however, they share little sequence similarity, although a few residues seem key to their interaction with fructose 6-phosphate.

Tomato soup is a soup with tomatoes as the primary ingredient. It can be served hot or cold, and may be made in a variety of ways. It may be smooth in texture, and there are also recipes that include chunks of tomato, cream, chicken or vegetable stock, vermicelli, chunks of other vegetables and meatballs. Many companies have their own versions of tomato soup which all vary in taste, portions and ingredients.
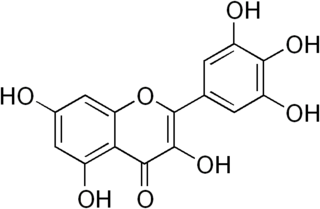
Myricetin is a member of the flavonoid class of polyphenolic compounds, with antioxidant properties. Common dietary sources include vegetables, fruits, nuts, berries, tea, and red wine. Myricetin is structurally similar to fisetin, luteolin, and quercetin and is reported to have many of the same functions as these other members of the flavonol class of flavonoids. Reported average intake of myricetin per day varies depending on diet, but has been shown in the Netherlands to average 23 mg/day.

Genistein (C15H10O5) is a naturally occurring compound that structurally belongs to a class of compounds known as isoflavones. It is described as an angiogenesis inhibitor and a phytoestrogen.

Fructose 1,6-bisphosphate, also known as Harden-Young ester, is fructose sugar phosphorylated on carbons 1 and 6. The β-D-form of this compound is common in cells. Upon entering the cell, most glucose and fructose is converted to fructose 1,6-bisphosphate.

Honokiol is a lignan isolated from the bark, seed cones, and leaves of trees belonging to the genus Magnolia. It has been identified as one of the chemical compounds in some traditional eastern herbal medicines along with magnolol, 4-O-methylhonokiol, and obovatol.

Homeobox protein Nkx-3.1, also known as NKX3-1, NKX3, BAPX2, NKX3A and NKX3.1 is a protein that in humans is encoded by the NKX3-1 gene located on chromosome 8p. NKX3-1 is a prostatic tumor suppressor gene.

α-Methylacyl-CoA racemase is an enzyme that in humans is encoded by the AMACR gene. AMACR catalyzes the following chemical reaction:

Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in in vitro and in vivo studies.

The TP53-inducible glycolysis and apoptosis regulator (TIGAR) also known as fructose-2,6-bisphosphatase TIGAR is an enzyme that in humans is encoded by the C12orf5 gene.

2-Hydroxyestradiol (2-OHE2), also known as estra-1,3,5(10)-triene-2,3,17β-triol, is an endogenous steroid, catechol estrogen, and metabolite of estradiol, as well as a positional isomer of estriol.