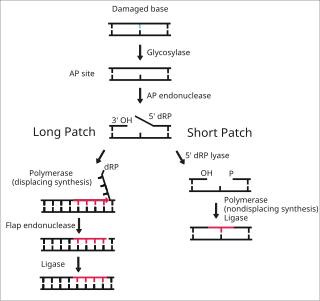
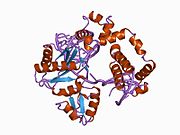
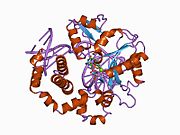
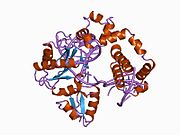
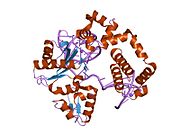
1bno: NMR SOLUTION STRUCTURE OF THE N-TERMINAL DOMAIN OF DNA POLYMERASE BETA, MINIMIZED AVERAGE STRUCTURE
1bnp: NMR SOLUTION STRUCTURE OF THE N-TERMINAL DOMAIN OF DNA POLYMERASE BETA, 55 STRUCTURES
1bpb: CRYSTAL STRUCTURE OF RAT DNA POLYMERASE BETA: EVIDENCE FOR A COMMON POLYMERASE MECHANISM
1bpd: CRYSTAL STRUCTURE OF RAT DNA POLYMERASE BETA: EVIDENCE FOR A COMMON POLYMERASE MECHANISM
1bpe: CRYSTAL STRUCTURE OF RAT DNA POLYMERASE BETA; EVIDENCE FOR A COMMON POLYMERASE MECHANISM
1bpx: DNA POLYMERASE BETA/DNA COMPLEX
1bpy: HUMAN DNA POLYMERASE BETA COMPLEXED WITH GAPPED DNA AND DDCTP
1bpz: HUMAN DNA POLYMERASE BETA COMPLEXED WITH NICKED DNA
1dk2: REFINED SOLUTION STRUCTURE OF THE N-TERMINAL DOMAIN OF DNA POLYMERASE BETA
1dk3: REFINED SOLUTION STRUCTURE OF THE N-TERMINAL DOMAIN OF DNA POLYMERASE BETA
1huo: CRYSTAL STRUCTURE OF DNA POLYMERASE BETA COMPLEXED WITH DNA AND CR-TMPPCP
1huz: CRYSTAL STRUCTURE OF DNA POLYMERASE COMPLEXED WITH DNA AND CR-PCP
1jn3: FIDELITY PROPERTIES AND STRUCTURE OF M282L MUTATOR MUTANT OF DNA POLYMERASE: SUBTLE STRUCTURAL CHANGES INFLUENCE THE MECHANISM OF NUCLEOTIDE DISCRIMINATION
1mq2: Human DNA Polymerase Beta Complexed With Gapped DNA Containing an 8-oxo-7,8-dihydro-Guanine and dAMP
1mq3: Human DNA Polymerase Beta Complexed With Gapped DNA Containing an 8-oxo-7,8-dihydro-Guanine Template Paired with dCTP
1nom: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7), 31-KD DOMAIN; SOAKED IN THE PRESENCE OF MNCL2 (5 MILLIMOLAR)
1rpl: 2.3 ANGSTROMS CRYSTAL STRUCTURE OF THE CATALYTIC DOMAIN OF DNA POLYMERASE BETA
1tv9: HUMAN DNA POLYMERASE BETA COMPLEXED WITH NICKED DNA CONTAINING A MISMATCHED TEMPLATE ADENINE AND INCOMING CYTIDINE
1tva: HUMAN DNA POLYMERASE BETA COMPLEXED WITH NICKED DNA CONTAINING A MISMATCHED TEMPLATE THYMIDINE AND INCOMING CYTIDINE
1zjm: Human DNA Polymerase beta complexed with DNA containing an A-A mismatched primer terminus
1zjn: Human DNA Polymerase beta complexed with DNA containing an A-A mismatched primer terminus with dGTP
1zqa: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF KCL (150 MILLIMOLAR) AT PH 7.5
1zqb: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF BACL2 (150 MILLIMOLAR)
1zqc: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF CACL2 (15 MILLIMOLAR)
1zqd: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF CACL2 (150 MILLIMOLAR)
1zqe: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF CRCL3 (SATURATED SOLUTION)
1zqf: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF CSCL (150 MILLIMOLAR)
1zqg: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF A SODIUM-FREE ARTIFICIAL MOTHER LIQUOR AT PH 6.5
1zqh: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF A SODIUM-FREE ARTIFICIAL MOTHER LIQUOR AT PH 7.5
1zqi: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF KCL (150 MILLIMOLAR)
1zqj: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF CACL2 (15 MILLIMOLAR) AND MGCL2 (15 MILLIMOLAR)
1zqk: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF KCL (75 MILLIMOLAR) AND MGCL2 (75 MILLIMOLAR)
1zql: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF MNCL2 (15 MILLIMOLAR) AND MGCL2 (15 MILLIMOLAR)
1zqm: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF MNCL2 (15 MILLIMOLAR)
1zqn: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF BACL2 (15 MILLIMOLAR) AND NACL (15 MILLIMOLAR)
1zqo: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF CACL2 (15 MILLIMOLAR) AND NACL (15 MILLIMOLAR)
1zqp: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF KCL (75 MILLIMOLAR) AND NACL (75 MILLIMOLAR)
1zqq: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF MNCL2 (15 MILLIMOLAR) AND NACL (15 MILLIMOLAR)
1zqr: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF NICL2
1zqs: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF TLCL (0.5 MILLIMOLAR)
1zqt: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (0.01 MILLIMOLAR) AND ZNCL2 (0.02 MILLIMOLAR)
1zqu: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7), 31-KD DOMAIN; SOAKED IN THE PRESENCE OF ARTIFICIAL MOTHER LIQUOR
1zqv: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7), 31-KD DOMAIN; SOAKED IN THE PRESENCE OF CACL2 (150 MILLIMOLAR)
1zqw: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7), 31-KD DOMAIN; SOAKED IN THE PRESENCE OF CSCL (150 MILLIMOLAR)
1zqx: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7), 31-KD DOMAIN; SOAKED IN THE PRESENCE OF KCL (150 MILLIMOLAR)
1zqy: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7), 31-KD DOMAIN; SOAKED IN THE PRESENCE OF MGCL2 (50 MILLIMOLAR)
1zqz: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7), 31-KD DOMAIN; SOAKED IN THE PRESENCE OF MNCL2 (50 MILLIMOLAR)
2bpc: CRYSTAL STRUCTURE OF RAT DNA POLYMERASE BETA: EVIDENCE FOR A COMMON POLYMERASE MECHANISM
2bpf: STRUCTURES OF TERNARY COMPLEXES OF RAT DNA POLYMERASE BETA, A DNA TEMPLATE-PRIMER, AND DDCTP
2bpg: STRUCTURES OF TERNARY COMPLEXES OF RAT DNA POLYMERASE BETA, A DNA TEMPLATE-PRIMER, AND DDCTP
2fmp: DNA Polymerase beta with a terminated gapped DNA substrate and ddCTP with sodium in the catalytic site
2fmq: Sodium in active site of DNA Polymerase Beta
2fms: DNA Polymerase beta with a gapped DNA substrate and dUMPNPP with magnesium in the catalytic site
2i9g: DNA Polymerase Beta with a Benzo[c]phenanthrene diol epoxide adducted guanine base
2iso: Ternary complex of DNA Polymerase beta with a dideoxy terminated primer and 2'-deoxyguanosine 5'-beta, gamma-difluoromethylene triphosphate
2isp: Ternary complex of DNA Polymerase beta with a dideoxy terminated primer and 2'-deoxyguanosine 5'-beta, gamma-methylene triphosphate
2p66: Human DNA Polymerase beta complexed with tetrahydrofuran (abasic site) containing DNA
7ice: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF CACL2
7icf: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF CDCL2 (0.1 MILLIMOLAR) (FOUR-DAY SOAK)
7icg: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF CDCL2
7ich: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF COCL2
7ici: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF CRCL3 (0.1 MILLIMOLAR)
7icj: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF CUCL2 (0.1 MILLIMOLAR)
7ick: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF MGCL2
7icl: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF MNCL2 (0.1 MILLIMOLAR)
7icm: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF MNCL2 (1.0 MILLIMOLAR)
7icn: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF NICL2
7ico: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF ZNCL2
7icp: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF ZNCL2 (0.01 MILLIMOLAR)
7icq: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF ZNCL2
7icr: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF ZNCL2
7ics: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF ZNCL2
7ict: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF ZNCL2 AND MGCL2
7icu: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF CDCL2 (0.1 MILLIMOLAR)
7icv: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF MNCL2 (0.1 MILLIMOLAR) AND IN THE ABSENCE OF NACL
8ica: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (1 MILLIMOLAR) AND CACL2 (5 MILLIMOLAR)
8icb: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF ARTIFICIAL MOTHER LIQUOR
8icc: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA (NO 5'-PHOSPHATE)
8ice: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (1 MILLIMOLAR) AND CDCL2 (1 MILLIMOLAR)
8icf: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (10 MILLIMOLAR) AND MGCL2 (50 MILLIMOLAR)
8icg: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (1 MILLIMOLAR) AND MGCL2 (5 MILLIMOLAR)
8ich: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DCTP (1 MILLIMOLAR) AND MGCL2 (5 MILLIMOLAR)
8ici: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DGTP (1 MILLIMOLAR) AND MGCL2 (5 MILLIMOLAR)
8icj: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX + THYMIDINE-5'-TRIPHOSPHATE, SOAKED IN THE PRESENCE OF DTTP AND MGCL2
8ick: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (1 MILLIMOLAR), MGCL2 (5 MILLIMOLAR), AND MNCL2 (5 MILLIMOLAR)
8icl: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (1 MILLIMOLAR) AND NICL2 (5 MILLIMOLAR)
8icm: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (1 MILLIMOLAR), MNCL2 (5 MILLIMOLAR), AND AMMONIUM SULFATE (75 MILLIMOLAR)
8icn: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF ATP (1 MILLIMOLAR) AND MNCL2 (5 MILLIMOLAR)
8ico: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF AZT-TP (1 MILLIMOLAR) AND MNCL2 (5 MILLIMOLAR)
8icp: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (1 MILLIMOLAR) AND MNCL2 (5 MILLIMOLAR)
8icq: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (0.1 MILLIMOLAR) AND MNCL2 (0.5 MILLIMOLAR)
8icr: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (1 MILLIMOLAR) AND MNCL2 (5 MILLIMOLAR)
8ics: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DCTP (1 MILLIMOLAR) AND MNCL2 (5 MILLIMOLAR)
8ict: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DCTP (1 MILLIMOLAR) AND MNCL2 (5 MILLIMOLAR)
8icu: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DDATP (1 MILLIMOLAR) AND MNCL2 (5 MILLIMOLAR)
8icv: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DGTP (1 MILLIMOLAR) AND MNCL2 (5 MILLIMOLAR)
8icw: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DTTP (1 MILLIMOLAR) AND MNCL2 (5 MILLIMOLAR)
8icx: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DTTP (1 MILLIMOLAR) AND MNCL2 (5 MILLIMOLAR)
8icy: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX + THYMIDINE-5'-TRIPHOSPHATE, SOAKED IN THE PRESENCE OF DTTP AND MNCL2
8icz: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF OF DATP (1 MILLIMOLAR), MNCL2 (5 MILLIMOLAR), AND LITHIUM SULFATE (75 MILLIMOLAR)
9ica: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX + 2'-DEOXYADENOSINE-5'-O-(1-THIOTRIPHOSPHATE), SOAKED IN THE PRESENCE OF DATP(ALPHA)S AND MNCL2
9icb: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX + 2'-DEOXYADENOSINE-5'-TRIPHOSPHATE, SOAKED IN THE PRESENCE OF DATP AND COCL2
9icc: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX + 2'-DEOXYADENOSINE-5'-TRIPHOSPHATE, SOAKED IN THE PRESENCE OF DATP AND CRCL3
9ice: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (1 MILLIMOLAR) AND CUCL2 (0.1 MILLIMOLAR)
9icf: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX + 2'-DEOXYADENOSINE-5'-TRIPHOSPHATE, SOAKED IN THE PRESENCE OF DATP AND ZNCL2
9icg: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DCTP (1 MILLIMOLAR) AND ZNCL2 (1 MILLIMOLAR)
9ich: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DGTP (1 MILLIMOLAR) AND ZNCL2 (1 MILLIMOLAR)
9ici: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DTTP (1 MILLIMOLAR) AND ZNCL2 (1 MILLIMOLAR)
9icj: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA
9ick: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF ARTIFICIAL MOTHER LIQUOR
9icl: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF PYROPHOSPHATE AND MNCL2
9icm: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DOUBLE STRANDED DNA (NO 5'-PHOSPHATE)
9icn: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX + 2',3'-DIDEOXYCYTIDINE-5'-TRIPHOSPHATE, SOAKED IN THE PRESENCE OF DDCTP AND MGCL2
9ico: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX, SOAKED IN THE PRESENCE OF DTTP AND MGCL2
9icp: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF PYROPHOSPHATE (1 MILLIMOLAR) AND MGCL2 (5 MILLIMOLAR)
9icq: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DATP (1 MILLIMOLAR) AND MNCL2 (5 MILLIMOLAR)
9icr: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX + 2'-DEOXYCYTIDINE-5'-TRIPHOSPHATE, SOAKED IN THE PRESENCE OF DCTP AND MNCL2
9ics: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX + 2',3'-DIDEOXYCYTIDINE-5'-TRIPHOSPHATE, SOAKED IN THE PRESENCE OF DDCTP AND MNCL2
9ict: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX + 2'-DEOXYGUANOSINE-5'-TRIPHOSPHATE, SOAKED IN THE PRESENCE OF DGTP AND MNCL2
9icu: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA; SOAKED IN THE PRESENCE OF DTTP (1 MILLIMOLAR) AND MNCL2 (5 MILLIMOLAR)
9icv: DNA POLYMERASE BETA (E.C.2.7.7.7)/DNA COMPLEX + 2'-DEOXYADENOSINE-5'-TRIPHOSPHATE, SOAKED IN THE PRESENCE OF DATP AND ZNCL2
9icw: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA; NATIVE STRUCTURE
9icx: DNA POLYMERASE BETA (POL B) (E.C.2.7.7.7) COMPLEXED WITH SIX BASE PAIRS OF DNA (NON GAPPED DNA ONLY)
9icy: DNA POLYMERASE BETA (E.C.2.7.7.7) COMPLEXED WITH SEVEN BASE PAIRS OF DNA (NON GAPPED DNA ONLY)