Asexual reproduction is a type of reproduction that does not involve the fusion of gametes or change in the number of chromosomes. The offspring that arise by asexual reproduction from either unicellular or multicellular organisms inherit the full set of genes of their single parent and thus the newly created individual is genetically and physically similar to the parent or an exact clone of the parent. Asexual reproduction is the primary form of reproduction for single-celled organisms such as archaea and bacteria. Many eukaryotic organisms including plants, animals, and fungi can also reproduce asexually. In vertebrates, the most common form of asexual reproduction is parthenogenesis, which is typically used as an alternative to sexual reproduction in times when reproductive opportunities are limited. Komodo dragons and some monitor lizards can reproduce asexually.

The rotifers, commonly called wheel animals or wheel animalcules, make up a phylum of microscopic and near-microscopic pseudocoelomate animals.
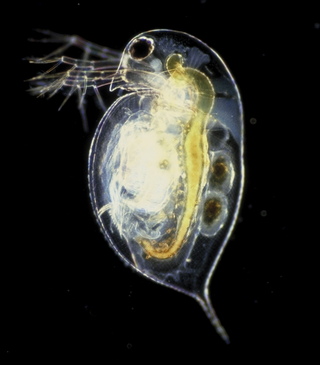
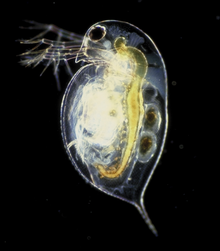
Daphnia is a genus of small planktonic crustaceans, 0.2–6.0 mm (0.01–0.24 in) in length. Daphnia are members of the order Anomopoda, and are one of the several small aquatic crustaceans commonly called water fleas because their saltatory swimming style resembles the movements of fleas. Daphnia spp. live in various aquatic environments ranging from acidic swamps to freshwater lakes and ponds.

Artemia is a genus of aquatic crustaceans also known as brine shrimp. It is the only genus in the family Artemiidae. The first historical record of the existence of Artemia dates back to the first half of the 10th century AD from Lake Urmia, Iran, with an example called by an Iranian geographer an "aquatic dog", although the first unambiguous record is the report and drawings made by Schlösser in 1757 of animals from Lymington, England. Artemia populations are found worldwide, typically in inland saltwater lakes, but occasionally in oceans. Artemia are able to avoid cohabiting with most types of predators, such as fish, by their ability to live in waters of very high salinity.

Thelytoky is a type of parthenogenesis and is the absence of mating and subsequent production of all female diploid offspring as for example in aphids. Thelytokous parthenogenesis is rare among animals and reported in about 1,500 species, about 1 in 1000 of described animal species, according to a 1984 study. It is more common in invertebrates, like arthropods, but it can occur in vertebrates, including salamanders, fish, and reptiles such as some whiptail lizards.

A lake ecosystem or lacustrine ecosystem includes biotic (living) plants, animals and micro-organisms, as well as abiotic (non-living) physical and chemical interactions. Lake ecosystems are a prime example of lentic ecosystems, which include ponds, lakes and wetlands, and much of this article applies to lentic ecosystems in general. Lentic ecosystems can be compared with lotic ecosystems, which involve flowing terrestrial waters such as rivers and streams. Together, these two ecosystems are examples of freshwater ecosystems.

Seed predation, often referred to as granivory, is a type of plant-animal interaction in which granivores feed on the seeds of plants as a main or exclusive food source, in many cases leaving the seeds damaged and not viable. Granivores are found across many families of vertebrates as well as invertebrates ; thus, seed predation occurs in virtually all terrestrial ecosystems. Seed predation is commonly divided into two distinctive temporal categories, pre-dispersal and post-dispersal predation, which affect the fitness of the parental plant and the dispersed offspring, respectively. Mitigating pre- and post-dispersal predation may involve different strategies. To counter seed predation, plants have evolved both physical defenses and chemical defenses. However, as plants have evolved seed defenses, seed predators have adapted to plant defenses. Thus, many interesting examples of coevolution arise from this dynamic relationship.

The American gizzard shad, also known as the mud shad, is a member of the herring family of fish and is native to large swaths of fresh and brackish waters in the United States of America, as well as portions of Quebec, Canada, and Mexico. The adult has a deep body, with a silvery-green coloration above fading to plain silver below. The gizzard shad commonly resides in freshwater lakes, reservoirs, rivers, and streams but can also reside in brackish waters, as it does on the Atlantic coast of the United States. Their range is across most of the continental United States, although they typically go no further north than New York and no further west than New Mexico. They are a large part of many of the ecosystems they inhabit and can drive changes in phyto- and zooplankton, thereby indirectly affecting other planktivorous fishes. The gizzard shad has been widely used as a food source for game fish, with varied success in management and effectiveness.

Parthenogenesis is a natural form of asexual reproduction in which growth and development of embryos occur in a gamete without combining with another gamete. In animals, parthenogenesis means development of an embryo from an unfertilized egg cell. In plants, parthenogenesis is a component process of apomixis. In algae, parthenogenesis can mean the development of an embryo from either an individual sperm or an individual egg.

The Diplostraca or Cladocera, commonly known as water fleas, is a superorder of small, mostly freshwater crustaceans, most of which feed on microscopic chunks of organic matter, though some forms are predatory.
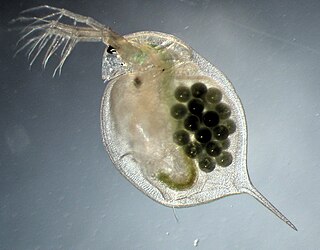
Daphnia magna is a small planktonic crustacean that belongs to the subclass Phyllopoda.

Leptodora is a genus containing two species of large, nearly transparent predatory water fleas. They grow up to 21 mm (0.83 in) long, with two large antennae used for swimming and a single compound eye. The legs are used to catch copepods that it comes into contact with by chance. Leptodora kindtii is found in temperate lakes across the Northern Hemisphere and is probably the only water flea species ever described in a newspaper; L. richardi is only known from eastern Russia. For most of the year, Leptodora reproduces parthenogenetically, with males only appearing late in the season, to produce winter eggs which hatch the following spring. Leptodora is the only genus in its family, the Leptodoridae, and suborder, Haplopoda.
Daphnia lumholtzi is a species of small, invasive water fleas that originates in the tropical and subtropical lakes of Africa, Asia, and Australia. As an invasive species, Daphnia lumholtzi disrupts aquatic habitats by spreading throughout the warmer waters of lakes and reservoirs.

Keratella cochlearis is a rotifer. The planktonic animal occurs worldwide in freshwater and marine habitats.

Notonecta maculata is a backswimmer of the family Notonectidae, found in Europe, including the United Kingdom.
Carla Cáceres is a professor at the University of Illinois Urbana-Champaign known for her research in population, community and evolutionary ecology, focusing on the origins, maintenance, and functional significance of biodiversity within ecosystems. She is a Fellow of the American Association for the Advancement of Science, the Ecological Society of America, and the Association for the Sciences of Limnology and Oceanography
Parthenogenesis is a mode of asexual reproduction in which offspring are produced by females without the genetic contribution of a male. Among all the sexual vertebrates, the only examples of true parthenogenesis, in which all-female populations reproduce without the involvement of males, are found in squamate reptiles. There are about 50 species of lizard and 1 species of snake that reproduce solely through parthenogenesis. It is unknown how many sexually reproducing species are also capable of parthenogenesis in the absence of males, but recent research has revealed that this ability is widespread among squamates.
Parthenogenesis is a form of reproduction where eggs develop without fertilization, resulting in unisexual species. This phenomenon is closely related with reproductive modes such as hybridogenesis, where fertilization occurs, but the paternal DNA is not passed on. Among amphibians, it is seen in numerous frog and salamander species, but has not been recorded in caecilians.

Egg predation is a feeding strategy in many groups of animals (ovivores) in which they consume eggs. Since an egg represents a complete organism at one stage of its life cycle, eating an egg is a form of predation, the killing of another organism for food.
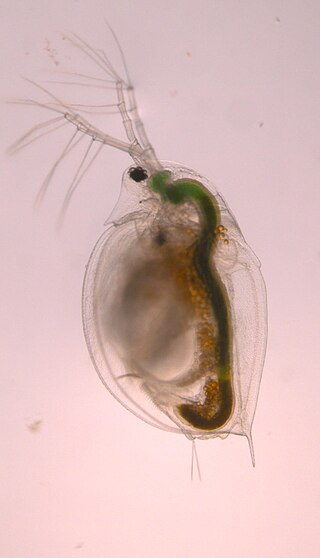
Daphnia pulicaria is a species of freshwater crustaceans found within the genus of Daphnia, which are often called "water fleas," and they are commonly used as model organisms for scientific research Like other species of Daphnia, they reproduce via cyclic parthenogenesis. D. pulicaria are filter-feeders with a diet primarily consisting of algae, including Ankistrodesmus falcatus, and they can be found in deep lakes located in temperate climates. Furthermore, D. pulicaria are ecologically important herbivorous zooplankton, which help control algal populations and are a source of food for some fish. D. pulicaria are closely related to Daphnia pulex, and numerous studies have investigated the nature and strength of this relationship because these species can produce Daphnia pulex-pulicaria hybrids. In recent years, D. pulicaria along with other Daphnia species have been negatively affected by invasive predators, such as Bythotrephes longimanus.