Related Research Articles

Reelin, encoded by the RELN gene, is a large secreted extracellular matrix glycoprotein that helps regulate processes of neuronal migration and positioning in the developing brain by controlling cell–cell interactions. Besides this important role in early development, reelin continues to work in the adult brain. It modulates synaptic plasticity by enhancing the induction and maintenance of long-term potentiation. It also stimulates dendrite and dendritic spine development and regulates the continuing migration of neuroblasts generated in adult neurogenesis sites like the subventricular and subgranular zones. It is found not only in the brain but also in the liver, thyroid gland, adrenal gland, fallopian tube, breast and in comparatively lower levels across a range of anatomical regions.

Magnetoreception is a sense which allows an organism to detect the Earth's magnetic field. Animals with this sense include some arthropods, molluscs, and vertebrates. The sense is mainly used for orientation and navigation, but it may help some animals to form regional maps. Experiments on migratory birds provide evidence that they make use of a cryptochrome protein in the eye, relying on the quantum radical pair mechanism to perceive magnetic fields. This effect is extremely sensitive to weak magnetic fields, and readily disturbed by radio-frequency interference, unlike a conventional iron compass.
Katanin is a microtubule-severing AAA protein. It is named after the Japanese sword called a katana. Katanin is a heterodimeric protein first discovered in sea urchins. It contains a 60 kDa ATPase subunit, encoded by KATNA1, which functions to sever microtubules. This subunit requires ATP and the presence of microtubules for activation. The second 80 kDA subunit, encoded by KATNB1, regulates the activity of the ATPase and localizes the protein to centrosomes. Electron microscopy shows that katanin forms 14–16 nm rings in its active oligomerized state on the walls of microtubules.

The Disabled-1 (Dab1) gene encodes a key regulator of Reelin signaling. Reelin is a large glycoprotein secreted by neurons of the developing brain, particularly Cajal-Retzius cells. DAB1 functions downstream of Reln in a signaling pathway that controls cell positioning in the developing brain and during adult neurogenesis. It docks to the intracellular part of the Reelin very low density lipoprotein receptor (VLDLR) and apoE receptor type 2 (ApoER2) and becomes tyrosine-phosphorylated following binding of Reelin to cortical neurons. In mice, mutations of Dab1 and Reelin generate identical phenotypes. In humans, Reelin mutations are associated with brain malformations and mental retardation. In mice, Dab1 mutation results in the scrambler mouse phenotype.

A reeler is a mouse mutant, so named because of its characteristic "reeling" gait. This is caused by the profound underdevelopment of the mouse's cerebellum, a segment of the brain responsible for locomotion. The mutation is autosomal and recessive, and prevents the typical cerebellar folia from forming.
Neurturin (NRTN) is a protein that is encoded in humans by the NRTN gene. Neurturin belongs to the glial cell line-derived neurotrophic factor (GDNF) family of neurotrophic factors, which regulate the survival and function of neurons. Neurturin’s role as a growth factor places it in the transforming growth factor beta (TGF-beta) subfamily along with its homologs persephin, artemin, and GDNF. It shares a 42% similarity in amino acid sequence with mature GDNF. It is also considered a trophic factor and critical in the development and growth of neurons in the brain. Neurotrophic factors like neurturin have been tested in several clinical trial settings for the potential treatment of neurodegenerative diseases, specifically Parkinson's disease.

CDKL5 is a gene that provides instructions for making a protein called cyclin-dependent kinase-like 5 also known as serine/threonine kinase 9 (STK9) that is essential for normal brain development. Mutations in the gene can cause deficiencies in the protein. The gene regulates neuronal morphology through cytoplasmic signaling and controlling gene expression. The CDKL5 protein acts as a kinase, which is an enzyme that changes the activity of other proteins by adding a cluster of oxygen and phosphorus atoms at specific positions. Researchers are currently working to determine which proteins are targeted by the CDKL5 protein.
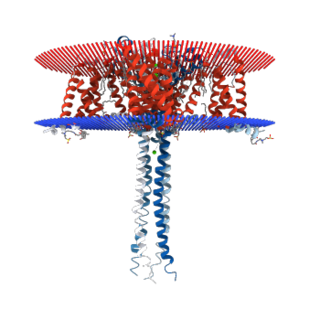
N-type calcium channels also called Cav2.2 channels are voltage gated calcium channels that are localized primarily on the nerve terminals and dendrites as well as neuroendocrine cells. The calcium N-channel consists of several subunits: the primary subunit α1B and the auxiliary subunits α2δ and β. The α1B subunit forms the pore through which the calcium enters and helps to determine most of the channel's properties. These channels play an important role in the neurotransmission during development. In the adult nervous system, N-type calcium channels are critically involved in the release of neurotransmitters, and in pain pathways. N-type calcium channels are the target of ziconotide, the drug prescribed to relieve intractable cancer pain. There are many known N-type calcium channel blockers that function to inhibit channel activity, although the most notable blockers are ω-conotoxins.

Low-density lipoprotein receptor-related protein 8 (LRP8), also known as apolipoprotein E receptor 2 (ApoER2), is a protein that in humans is encoded by the LRP8 gene. ApoER2 is a cell surface receptor that is part of the low-density lipoprotein receptor family. These receptors function in signal transduction and endocytosis of specific ligands. Through interactions with one of its ligands, reelin, ApoER2 plays an important role in embryonic neuronal migration and postnatal long-term potentiation. Another LDL family receptor, VLDLR, also interacts with reelin, and together these two receptors influence brain development and function. Decreased expression of ApoER2 is associated with certain neurological diseases.

Neuronal migration protein doublecortin, also known as doublin or lissencephalin-X is a protein that in humans is encoded by the DCX gene.

Class III β-tubulin, otherwise known as βIII-tubulin (β3-tubulin) or β-tubulin III, is a microtubule element of the tubulin family found almost exclusively in neurons, and in testis cells. In humans, it is encoded by the TUBB3 gene.

Kalirin, also known as Huntingtin-associated protein-interacting protein (HAPIP), protein duo (DUO), or serine/threonine-protein kinase with Dbl- and pleckstrin homology domain, is a protein that in humans is encoded by the KALRN gene. Kalirin was first identified in 1997 as a protein interacting with huntingtin-associated protein 1. Is also known to play an important role in nerve growth and axonal development.
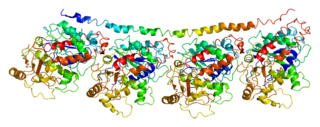
Tubulin alpha-1A chain is a protein that in humans is encoded by the TUBA1A gene.

T-box, brain, 1 is a transcription factor protein important in vertebrate embryo development. It is encoded by the TBR1 gene. This gene is also known by several other names: T-Brain 1, TBR-1, TES-56, and MGC141978. TBR1 is a member of the TBR1 subfamily of T-box family transcription factors, which share a common DNA-binding domain. Other members of the TBR1 subfamily include EOMES and TBX21. TBR1 is involved in the differentiation and migration of neurons and is required for normal brain development. TBR1 interacts with various genes and proteins in order to regulate cortical development, specifically within layer VI of the developing six-layered human cortex. Studies show that TBR1 may play a role in major neurological diseases such as Alzheimer's disease (AD), Parkinson's disease (PD) and autism spectrum disorder (ASD).

Kinesin-like protein KIF1A, also known as axonal transporter of synaptic vesicles or microtubule-based motor KIF1A, is a protein that in humans is encoded by the KIF1A gene.

Alcino J. Silva is a Portuguese-American neuroscientist who was the recipient of the 2008 Order of Prince Henry and elected as a fellow of the American Association for the Advancement of Science in 2013 for his contributions to the molecular cellular cognition of memory, a field he pioneered with the publication of two articles in Science in 1992.
Cajal–Retzius cells are a heterogeneous population of morphologically and molecularly distinct reelin-producing cell types in the marginal zone/layer I of the developmental cerebral cortex and in the immature hippocampus of different species and at different times during embryogenesis and postnatal life.

Neurotubules are microtubules found in neurons in nervous tissues. Along with neurofilaments and microfilaments, they form the cytoskeleton of neurons. Neurotubules are undivided hollow cylinders that are made up of tubulin protein polymers and arrays parallel to the plasma membrane in neurons. Neurotubules have an outer diameter of about 23 nm and an inner diameter, also known as the central core, of about 12 nm. The wall of the neurotubules is about 5 nm in width. There is a non-opaque clear zone surrounding the neurotubule and it is about 40 nm in diameter. Like microtubules, neurotubules are greatly dynamic and the length of them can be adjusted by polymerization and depolymerization of tubulin.

Mary Elizabeth Hatten is the Frederick P. Rose Professor of neuroscience at the Rockefeller University, where she became the first female full professor in 1992. She studies the manner in which neurons migrate in the brain, which has implications for many neurological diseases, as well as cancer. Her research led to her being elected to the National Academy of Sciences in 2017.
Erich Pascal Malkemper is a German neuroscientist. He studies magnetoreception and animal hearing and he is currently a group leader of the Max Planck Society at the Center of Advanced European Studies and Research (CAESAR) in Bonn, Germany.
References
- ↑ Sandall, D. W.; Satkunanathan, N.; Keays, D. A.; Polidano, M. A.; Liping, X.; Pham, V.; Down, J. G.; Khalil, Z.; Livett, B. G.; Gayler, K. R. (2003). "A Novel α-Conotoxin Identified by Gene Sequencing is Active in Suppressing the Vascular Response to Selective Stimulation of Sensory Nerves in Vivo†". Biochemistry. 42 (22): 6904–6911. doi:10.1021/bi034043e. PMID 12779345.
- ↑ Keays, David A.; Tian, Guoling; Poirier, Karine; Huang, Guo-Jen; Siebold, Christian; Cleak, James; Oliver, Peter L.; Fray, Martin; Harvey, Robert J.; Molnár, Zoltán; Piñon, Maria C.; Dear, Neil; Valdar, William; Brown, Steve D.M.; Davies, Kay E.; Rawlins, J. Nicholas P.; Cowan, Nicholas J.; Nolan, Patrick; Chelly, Jamel; Flint, Jonathan (2007). "Mutations in α-Tubulin Cause Abnormal Neuronal Migration in Mice and Lissencephaly in Humans". Cell. 128 (1): 45–57. doi:10.1016/j.cell.2006.12.017. PMC 1885944 . PMID 17218254.
- ↑ Treiber, Christoph Daniel; Salzer, Marion Claudia; Riegler, Johannes; Edelman, Nathaniel; Sugar, Cristina; Breuss, Martin; Pichler, Paul; Cadiou, Herve; Saunders, Martin; Lythgoe, Mark; Shaw, Jeremy; Keays, David Anthony (2012). "Clusters of iron-rich cells in the upper beak of pigeons are macrophages not magnetosensitive neurons". Nature. 484 (7394): 367–370. Bibcode:2012Natur.484..367T. doi:10.1038/nature11046. PMID 22495303. S2CID 205228624.
- ↑ Edelman, NB., Fritz, T., Nimpf, S., Pichler, P., Lauwers, M., Hickman, RW., Papadaki-Anastasopoulou, A., Ushakova, L., Heuser, T., Resch, GP., Saunders, M., Shaw, JA., Keays, DA. (2015). No evidence for intracellular magnetite in putative vertebrate magnetoreceptors identified by magnetic screening. Proc Natl Acad Sci U S A. 112(1):262-7
- ↑ Nimpf S, Nordmann GC, Kagerbauer D, Malkemper EP, Landler L, Papadaki-Anastasopoulou A, Ushakova L, Wenninger-Weinzierl A, Novatchkova M, Vincent P, Lendl T, Colombini M, Mason MJ, Keays DA. A putative mechanism for magnetoreception by electromagnetic induction in the pigeon inner ear. Curr Biol. 2019 Dec 2;29(23):4052-4059
- ↑ Breuss, M., Heng, JI., Poirier, K., Tian, G., Jaglin, XH., Qu, Z., Braun, A., Gstrein, T., Ngo, L., Haas, M., Bahi-Buisson, N., Moutard, ML., Passemard, S., Verloes, A., Gressens, P., Xie, Y., Robson, KJ., Rani, DS., Thangaraj, K., Clausen, T., Chelly, J., Cowan, NJ., Keays, DA. (2012). Mutations in the β-tubulin gene TUBB5 cause microcephaly with structural brain abnormalities. Cell Rep. 2(6):1554-62
- ↑ Tripathy R, van Dijk T, van Bon B, Gstrein T, Bahi-Buisson N, Paciorkowski A, Pagnamenta A, Taylor J, Terrone G, Vitiello G, D’Amico A, Del Giudice E, Brunetti-Pierri N, Reymond A, Voisin N, Bernstein JA, Farrelly E, Pierson T, Kini U, Leonard T, Mirzaa G, Baas F, Chelly J, Keays DA. Mutations in MAST1 cause mega corpus callosum syndrome and cortical malformations. Neuron. 2018 Dec 19;100(6):1354-1368.
- ↑ Gstrein T, Edwards A, Přistoupilová A, Leca I, Breuss M, Pilat-Carotta S, Hansen AH, Tripathy R, Traunbauer AK, Hochstoeger T, Rosoklija G, Repic M, Landler L, Stránecký V, Dürnberger G, Keane TM, Zuber J, Adams DJ, Flint J, Honzik T, Gut M, Beltran S, Mechtler K, Sherr E, Kmoch S, Gut I, Keays DA. (2018). Mutations in Vps15 perturb neuronal migration in mice and are associated with neurodevelopmental disease in humans. Nature Neuroscience. Feb;21(2):207-217
- ↑ "People | keays lab".
- ↑ "BBC Two - David Attenborough's Natural Curiosities, Series 4, Finding the Way".
- ↑ "Columbarian Columbuses". The Economist. 13 July 2013.
- ↑ "How do Animals Keep from Getting Lost?". The New Yorker . 28 May 2016.
- ↑ "BBC Radio 4 - Show Me the Way to Go Home".
- ↑ "Iron found in bird brains could be key to migration". NBC News . 6 May 2013.
- ↑ "Pigeon riddle has scientists in a flap". ABC News. 11 April 2012.