Rhadinovirus is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Gammaherpesvirinae. Humans and other mammals serve as natural hosts. There are 12 species in this genus. Diseases associated with this genus include: Kaposi's sarcoma, primary effusion lymphoma and multicentric Castleman's disease, caused by Human gammaherpesvirus 8 (HHV-8), also known as Kaposi's sarcoma-associated herpesvirus (KSHV). The term rhadino comes from the Latin fragile, referring to the tendency of the viral genome to break apart when it is isolated.

Kaposi's sarcoma-associated herpesvirus (KSHV) is the ninth known human herpesvirus; its formal name according to the International Committee on Taxonomy of Viruses (ICTV) is Human gammaherpesvirus 8, or HHV-8 in short. Like other herpesviruses, its informal names are used interchangeably with its formal ICTV name. This virus causes Kaposi's sarcoma, a cancer commonly occurring in AIDS patients, as well as primary effusion lymphoma, HHV-8-associated multicentric Castleman's disease and KSHV inflammatory cytokine syndrome. It is one of seven currently known human cancer viruses, or oncoviruses. Even after many years since the discovery of KSHV/HHV8, there is no known cure for KSHV associated tumorigenesis.
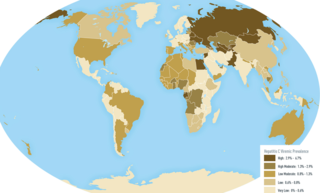
An oncovirus or oncogenic virus is a virus that can cause cancer. This term originated from studies of acutely transforming retroviruses in the 1950–60s, when the term "oncornaviruses" was used to denote their RNA virus origin. With the letters "RNA" removed, it now refers to any virus with a DNA or RNA genome causing cancer and is synonymous with "tumor virus" or "cancer virus". The vast majority of human and animal viruses do not cause cancer, probably because of longstanding co-evolution between the virus and its host. Oncoviruses have been important not only in epidemiology, but also in investigations of cell cycle control mechanisms such as the retinoblastoma protein.
Primary effusion lymphoma (PEL) is classified as a diffuse large B cell lymphoma. It is a rare malignancy of plasmablastic cells that occurs in individuals that are infected with the Kaposi's sarcoma-associated herpesvirus. Plasmablasts are immature plasma cells, i.e. lymphocytes of the B-cell type that have differentiated into plasmablasts but because of their malignant nature do not differentiate into mature plasma cells but rather proliferate excessively and thereby cause life-threatening disease. In PEL, the proliferating plasmablastoid cells commonly accumulate within body cavities to produce effusions, primarily in the pleural, pericardial, or peritoneal cavities, without forming a contiguous tumor mass. In rare cases of these cavitary forms of PEL, the effusions develop in joints, the epidural space surrounding the brain and spinal cord, and underneath the capsule which forms around breast implants. Less frequently, individuals present with extracavitary primary effusion lymphomas, i.e., solid tumor masses not accompanied by effusions. The extracavitary tumors may develop in lymph nodes, bone, bone marrow, the gastrointestinal tract, skin, spleen, liver, lungs, central nervous system, testes, paranasal sinuses, muscle, and, rarely, inside the vasculature and sinuses of lymph nodes. As their disease progresses, however, individuals with the classical effusion-form of PEL may develop extracavitary tumors and individuals with extracavitary PEL may develop cavitary effusions.

Robert Yarchoan is a medical researcher who played an important role in the development of the first effective drugs for AIDS. He is the Chief of the HIV and AIDS Malignancy Branch in the NCI and also coordinates HIV/AIDS malignancy research throughout the NCI as director of the Office of HIV and AIDS Malignancy (OHAM).

Patrick S. Moore is an Irish and American virologist and epidemiologist who co-discovered together with his wife, Yuan Chang, two different human viruses causing the AIDS-related cancer Kaposi's sarcoma and the skin cancer Merkel cell carcinoma. The couple met while in medical school together and were married in 1989 while they pursued fellowships at different universities.

Yuan Chang is a Taiwanese-American virologist and pathologist who co-discovered together with her husband, Patrick S. Moore, the Kaposi's sarcoma-associated herpesvirus (KSHV) and Merkel cell polyomavirus, two of the seven known human oncoviruses.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.

Herpes simplex virus1 and 2, also known by their taxonomic names Human alphaherpesvirus 1 and Human alphaherpesvirus 2, are two members of the human Herpesviridae family, a set of viruses that produce viral infections in the majority of humans. Both HSV-1 and HSV-2 are very common and contagious. They can be spread when an infected person begins shedding the virus.

The University of Virginia School of Medicine is the graduate medical school of the University of Virginia. The school's facilities are on the University of Virginia grounds adjacent to Academical Village in Charlottesville, Virginia. Founded in 1819 by Thomas Jefferson, UVA SoM is the tenth oldest medical school in the United States. The School of Medicine confers Doctor of Medicine (M.D.) and Doctor of Philosophy (PhD) degrees, and is closely associated with both the University of Virginia Health System and Inova Health System.

Gammaherpesvirinae is a subfamily of viruses in the order Herpesvirales and in the family Herpesviridae. Viruses in Gammaherpesvirinae are distinguished by reproducing at a more variable rate than other subfamilies of Herpesviridae. Mammals serve as natural hosts. There are 43 species in this subfamily, divided among 7 genera with three species unassigned to a genus. Diseases associated with this subfamily include: HHV-4: infectious mononucleosis. HHV-8: Kaposi's sarcoma.
Murid gammaherpesvirus 68 (MuHV-68) is an isolate of the virus species Murid gammaherpesvirus 4, a member of the genus Rhadinovirus. It is a member of the subfamily Gammaherpesvirinae in the family of Herpesviridae. MuHV-68 serves as a model for study of human gammaherpesviruses which cause significant human disease including B-cell lymphoma and Kaposi's sarcoma. The WUMS strain of MuHV-68 was fully sequenced and annotated in 1997, and the necessity of most of its genes in viral replication was characterized by random transposon mutagenesis.
The latency-associated nuclear antigen (LANA-1) or latent nuclear antigen is a Kaposi's sarcoma-associated herpesvirus (KSHV) latent protein initially found by Moore and colleagues as a speckled nuclear antigen present in primary effusion lymphoma cells that reacts with antibodies from patients with KS. It is the most immunodominant KSHV protein identified by Western-blotting as 222–234 kDa double bands migrate slower than the predicted molecular weight. LANA has been suspected of playing a crucial role in modulating viral and cellular gene expression. It is commonly used as an antigen in blood tests to detect antibodies in persons that have been exposed to KSHV.

Kaposi's sarcoma (KS) is a type of cancer that can form masses in the skin, in lymph nodes, in the mouth, or in other organs. The skin lesions are usually painless, purple and may be flat or raised. Lesions can occur singly, multiply in a limited area, or may be widespread. Depending on the sub-type of disease and level of immune suppression, KS may worsen either gradually or quickly. Except for Classical KS where there is generally no immune suppression, KS is caused by a combination of immune suppression and infection by Human herpesvirus 8.
Large B-cell lymphoma arising in HHV8-associated multicentric Castleman's disease is a type of large B-cell lymphoma, recognized in the WHO 2008 classification. It is sometimes called the plasmablastic form of multicentric Castleman disease. It has sometimes been confused with plasmablastic lymphoma in the literature, although that is a dissimilar specific entity. It has variable CD20 expression and unmutated immunoglobulin variable region genes.
Eva Henriette Gottwein is a virologist and Associate Professor of Microbiology-Immunology at Northwestern University Feinberg School of Medicine in Chicago, Illinois. The main focus of her research is the role of viral miRNAs involved in herpesviral oncogenesis. Gottwein is member of Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Her contributions as a member include the focus on how encoded miRNAs target and function in the human oncogenic herpesvirus Kaposi's sarcoma-associated herpesvirus known as KSHV.
Human herpes viruses, also known as HHVs, are part of a family of DNA viruses that cause several diseases in humans. One of the most notable functions of this virus family is their ability to enter a latent phase and lay dormant within animals for extended periods of time. The mechanism that controls this is very complex because expression of viral proteins during latency is decreased a great deal, meaning that the virus must have transcription of its genes repressed. There are many factors and mechanisms that control this process and epigenetics is one way this is accomplished. Epigenetics refers to persistent changes in expression patterns that are not caused by changes to the DNA sequence. This happens through mechanisms such as methylation and acetylation of histones, DNA methylation, and non-coding RNAs (ncRNA). Altering the acetylation of histones creates changes in expression by changing the binding affinity of histones to DNA, making it harder or easier for transcription machinery to access the DNA. Methyl and acetyl groups can also act as binding sites for transcription factors and enzymes that further modify histones or alter the DNA itself.
HSV epigenetics is the epigenetic modification of herpes simplex virus (HSV) genetic code.
Donald "Don" Emil Ganem is an American physician, virologist, professor emeritus of microbiology and medicine, and former global head of infectious disease research at Novartis Institutes for BioMedical Research (NIBR).
David John Blackbourn PhD FRSB is currently Professor of Virology and Director of the Institute of Medical Sciences at the University of Aberdeen. His research interests emphasise virus understanding how viruses have evolved to manipulate immune responses. Having previously been Head of the School of Biosciences and Medicine for 8 years. He is also a co-founder of Ducentis BioTherapeutics to produce a novel anti-inflammatory therapeutic drug and until September 2022, was non-executive board member. Blackbourn is an Editor-in-Chief of FEMS Microbiology Reviews.












