
In biology, mating is the pairing of either opposite-sex or hermaphroditic organisms for the purposes of sexual reproduction. Fertilization is the fusion of two gametes. Copulation is the union of the sex organs of two sexually reproducing animals for insemination and subsequent internal fertilization. Mating may also lead to external fertilization, as seen in amphibians, fishes and plants. For most species, mating is between two individuals of opposite sexes. However, for some hermaphroditic species, copulation is not required because the parent organism is capable of self-fertilization (autogamy); for example, banana slugs.

Sperm competition is the competitive process between spermatozoa of two or more different males to fertilize the same egg during sexual reproduction. Competition can occur when females have multiple potential mating partners. Greater choice and variety of mates increases a female's chance to produce more viable offspring. However, multiple mates for a female means each individual male has decreased chances of producing offspring. Sperm competition is an evolutionary pressure on males, and has led to the development of adaptations to increase male's chance of reproductive success. Sperm competition results in a sexual conflict between males and females. Males have evolved several defensive tactics including: mate-guarding, mating plugs, and releasing toxic seminal substances to reduce female re-mating tendencies to cope with sperm competition. Offensive tactics of sperm competition involve direct interference by one male on the reproductive success of another male, for instance by physically removing another male's sperm prior to mating with a female. For an example, see Gryllus bimaculatus.

Reproductive success is an individual's production of offspring per breeding event or lifetime. This is not limited by the number of offspring produced by one individual, but also the reproductive success of these offspring themselves.

Sexual conflict or sexual antagonism occurs when the two sexes have conflicting optimal fitness strategies concerning reproduction, particularly over the mode and frequency of mating, potentially leading to an evolutionary arms race between males and females. In one example, males may benefit from multiple matings, while multiple matings may harm or endanger females, due to the anatomical differences of that species. Sexual conflict underlies the evolutionary distinction between male and female.

The Neriidae are a family of true flies (Diptera) closely related to the Micropezidae. Some species are known as cactus flies, while others have been called banana stalk flies and the family was earlier treated as subfamily of the Micropezidae which are often called stilt-legged flies. Neriids differ from micropezids in having no significant reduction of the fore legs. Neriids breed in rotting vegetation, such as decaying tree bark or rotting fruit. About 100 species are placed in 19 genera. Neriidae are found mainly in tropical regions, but two North American genera occur, each with one species, and one species of Telostylinus occurs in temperate regions of eastern Australia.

Sexual cannibalism is when an animal, usually the female, cannibalizes its mate prior to, during, or after copulation. It is a trait observed in many arachnid orders and several insect and crustacean clades. Several hypotheses to explain this seemingly paradoxical behavior have been proposed. The adaptive foraging hypothesis, aggressive spillover hypothesis and mistaken identity hypothesis are among the proposed hypotheses to explain how sexual cannibalism evolved. This behavior is believed to have evolved as a manifestation of sexual conflict, occurring when the reproductive interests of males and females differ. In many species that exhibit sexual cannibalism, the female consumes the male upon detection. Females of cannibalistic species are generally hostile and unwilling to mate; thus many males of these species have developed adaptive behaviors to counteract female aggression.

Phormia regina, the black blow fly, belongs to the blow fly family Calliphoridae and was first described by Johann Wilhelm Meigen.

Scathophaga stercoraria, commonly known as the yellow dung fly or the golden dung fly, is one of the most familiar and abundant flies in many parts of the Northern Hemisphere. As its common name suggests, it is often found on the feces of large mammals, such as horses, cattle, sheep, deer, and wild boar, where it goes to breed. The distribution of S. stercoraria is likely influenced by human agriculture, especially in northern Europe and North America. The Scathophaga are integral in the animal kingdom due to their role in the natural decomposition of dung in fields. They are also very important in the scientific world due to their short life cycles and susceptibility to experimental manipulations; thus, they have contributed significant knowledge about animal behavior.
Bateman's principle, in evolutionary biology, is that in most species, variability in reproductive success is greater in males than in females. It was first proposed by Angus John Bateman (1919–1996), an English geneticist. Bateman suggested that, since males are capable of producing millions of sperm cells with little effort, while females invest much higher levels of energy in order to nurture a relatively small number of eggs, the female plays a significantly larger role in their offspring's reproductive success. Bateman's paradigm thus views females as the limiting factor of parental investment, over which males will compete in order to copulate successfully.
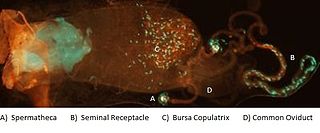
Female sperm storage is a biological process and often a type of sexual selection in which sperm cells transferred to a female during mating are temporarily retained within a specific part of the reproductive tract before the oocyte, or egg, is fertilized. This process takes place in some species of animals, but not in humans. The site of storage is variable among different animal taxa and ranges from structures that appear to function solely for sperm retention, such as insect spermatheca and bird sperm storage tubules, to more general regions of the reproductive tract enriched with receptors to which sperm associate before fertilization, such as the caudal portion of the cow oviduct containing sperm-associating annexins. Female sperm storage is an integral stage in the reproductive process for many animals with internal fertilization. It has several documented biological functions including:
Sexual antagonistic co-evolution is the relationship between males and females where sexual morphology changes over time to counteract the opposite's sex traits to achieve the maximum reproductive success. This has been compared to an arms race between sexes. In many cases, male mating behavior is detrimental to the female's fitness. For example, when insects reproduce by means of traumatic insemination, it is very disadvantageous to the female's health. During mating, males will try to inseminate as many females as possible, however, the more times a female's abdomen is punctured, the less likely she is to survive. Females that possess traits to avoid multiple matings will be more likely to survive, resulting in a change in morphology. In males, genitalia is relatively simple and more likely to vary among generations compared to female genitalia. This results in a new trait that females have to avoid in order to survive.
Interlocus sexual conflict is a type of sexual conflict that occurs through the interaction of a set of antagonistic alleles at two or more different loci, or the location of a gene on a chromosome, in males and females, resulting in the deviation of either or both sexes from the fitness optima for the traits. A co-evolutionary arms race is established between the sexes in which either sex evolves a set of antagonistic adaptations that is detrimental to the fitness of the other sex. The potential for reproductive success in one organism is strengthened while the fitness of the opposite sex is weakened. Interlocus sexual conflict can arise due to aspects of male–female interactions such as mating frequency, fertilization, relative parental effort, female remating behavior, and female reproductive rate.

Sepsis cynipsea is a European species of fly and member of the family Sepsidae. It is a coprophagous fly that feeds on dung. These flies are most commonly found around freshly laid cattle dung where they eat and reproduce. Due to human agricultural practices involving cows, these flies are now common in other areas of the world.
A nuptial gift is a nutritional gift given by one partner in some animals' sexual reproduction practices.

Sexual selection in amphibians involves sexual selection processes in amphibians, including frogs, salamanders and newts. Prolonged breeders, the majority of frog species, have breeding seasons at regular intervals where male-male competition occurs with males arriving at the waters edge first in large number and producing a wide range of vocalizations, with variations in depth of calls the speed of calls and other complex behaviours to attract mates. The fittest males will have the deepest croaks and the best territories, with females making their mate choices at least partly based on the males depth of croaking. This has led to sexual dimorphism, with females being larger than males in 90% of species, males in 10% and males fighting for groups of females.
Sexual coercion among animals is the use of violence, threats, harassment, and other tactics to help them forcefully copulate. Such behavior has been compared to sexual assault, including rape, among humans.
Cryptic female choice is a form of mate choice which occurs both in pre and post copulatory circumstances when females in certain species use physical or chemical mechanisms to control a male's success of fertilizing their ova or ovum; i.e. by selecting whether sperm are successful in fertilizing their eggs or not. It occurs in internally-fertilizing species and involves differential use of sperm by females when sperm are available in the reproductive tract.
Drosophila nigrospiracula is a fly species indigenous to the Sonoran Desert, spanning Arizona, Baja California, and part of Sonora, Mexico. D. nigrospiracula share the Sonoran Desert with three other species of Drosophila: D. pachea, D. mettleri, and D. mojavensis. This fly breeds on the decomposing tissues of two species of cacti that are also endemic to the region: cardón (Pachycereus pringlei) and saguaro (Carnegiea gigantea).
Glyphidops flavifrons is a member of the Neriidae family of the order Diptera. This fly is found in the southern United States, Central America, and South America. Historically, it has also been called Oncopsia seductrix Hennig or Oncopsia mexicana.G. flavifrons live, reproduce, and lay their eggs on the bark of trees in the early stages of decay. In this species, it is common to see the male flies to exhibit aggression in the presence of the females. These males may attack the copulating pair to help decrease the chances of other males mating and increase their own chances.

Prochyliza xanthostoma, the waltzing fly, is a species of carrion-feeding cheese skipper, insects in the family Piophilidae and the order Diptera. P. xanthostoma is a member of the genus Prochyliza, which contains eleven species. The adult flies are found through North America and are brown-bodied, with orange and black coloring. Mating occurs on animal carcasses and male perform mating rituals; females engage in ejaculate feeding. The waltzing fly is known for its exaggerated sexual dimorphism and has thus become a prominent model for sexual dimorphism and larval behavior. These organisms are known as cheese skippers because when startled, the larvae can leap several inches into the air. P. xanthostoma is an important model organism for sexual selection, larval behavior, and adult reproductive success and survivability.











