
Serotonin or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex, touching on diverse functions including mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction.

Serotonin syndrome (SS) is a group of symptoms that may occur with the use of certain serotonergic medications or drugs. The symptoms can range from mild to severe, and are potentially fatal. Symptoms in mild cases include high blood pressure and a fast heart rate; usually without a fever. Symptoms in moderate cases include high body temperature, agitation, increased reflexes, tremor, sweating, dilated pupils, and diarrhea. In severe cases, body temperature can increase to greater than 41.1 °C (106.0 °F). Complications may include seizures and extensive muscle breakdown.

Mirtazapine, sold under the brand name Remeron among others, is an atypical tetracyclic antidepressant, and as such is used primarily to treat depression. Its effects may take up to four weeks but can also manifest as early as one to two weeks. It is often used in cases of depression complicated by anxiety or insomnia. The effectiveness of mirtazapine is comparable to other commonly prescribed antidepressants. It is taken by mouth.
Mester de Clerecía is a Spanish literature genre that can be understood as an opposition and surpassing of Mester de Juglaría. It was cultivated in the 13th century by Spanish learned poets, usually clerics.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

5-Hydroxytryptophan (5-HTP), used medically as oxitriptan, is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitter serotonin.

4-Methylthioamphetamine (4-MTA) is a designer drug of the substituted amphetamine class developed in the 1990s by a team led by David E. Nichols, an American pharmacologist and medical chemist, at Purdue University. It acts as a non-neurotoxic highly selective serotonin releasing agent (SSRA) in animals. 4-MTA is the methylthio derivative of amphetamine.

Aralkylamine N-acetyltransferase (AANAT), also known as arylalkylamine N-acetyltransferase or serotonin N-acetyltransferase (SNAT), is an enzyme that is involved in the day/night rhythmic production of melatonin, by modification of serotonin. It is in humans encoded by the ~2.5 kb AANAT gene containing four exons, located on chromosome 17q25. The gene is translated into a 23 kDa large enzyme. It is well conserved through evolution and the human form of the protein is 80 percent identical to sheep and rat AANAT. It is an acetyl-CoA-dependent enzyme of the GCN5-related family of N-acetyltransferases (GNATs). It may contribute to multifactorial genetic diseases such as altered behavior in sleep/wake cycle and research is on-going with the aim of developing drugs that regulate AANAT function.

Praid is a commune in Harghita County, Romania. It lies in the Székely Land, an ethno-cultural region in eastern Transylvania, and is composed of six villages: Becaș (Békástanya), Bucin (Bucsin), Ocna de Jos (Alsósófalva), Ocna de Sus (Felsősófalva), Praid (Parajd), and Șașvereș (Sásverés).

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.
Jorge Mester is a Mexican conductor of Hungarian ancestry. He has served as the artistic director for the Orquesta Filarmónica de Boca del Río, Veracruz, since it was founded in 2014.

MDAI (5,6-methylenedioxy-2-aminoindane) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in vitro and produces entactogen effects in humans.
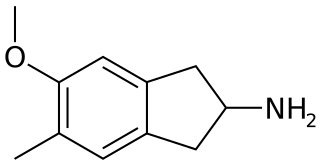
5-Methoxy-6-methyl-2-aminoindane (MMAI) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) and produces entactogen effects in humans. It has been sold as a designer drug and research chemical online since 2010.
A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons.

Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions.

3,4-Dichloroamphetamine (3,4-DCA), is an amphetamine derived drug invented by Eli Lilly in the 1960s, which has a number of pharmacological actions. It acts as a highly potent and selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with high affinity, but also acts as a selective serotonergic neurotoxin in a similar manner to the related para-chloroamphetamine, though with slightly lower potency. It is also a monoamine oxidase inhibitor (MAOI), as well as a very potent inhibitor of the enzyme phenylethanolamine N-methyl transferase which normally functions to transform noradrenaline into adrenaline in the body.

Arachidonoyl serotonin is an endogenous lipid signaling molecule. It was first described in 1998 as being an inhibitor of fatty acid amide hydrolase (FAAH). In 2007, it was shown to have analgesic properties and to act as an antagonist of the TRPV1 receptor. In 2011, it was shown to be present in the ileum and jejunum of the gastrointestinal tract and modulate glucagon-like peptide-1 (GLP-1) secretion. In addition to this, in 2016, AA-5-HT was also found to affect the signaling mechanisms responsible for anxiety, by inhibiting dopamine release from the Basolateral amygdala following fear behavior. In 2017, AA-5-HT was tested in its effects on the sleep wake cycle, where it was found to affect the sleep homeostasis when used in conjunction with molecules and chemicals that affect wake-related neurotransmitters.

Loretta J. Mester was president and CEO of the Federal Reserve Bank of Cleveland.

Amesergide is a serotonin receptor antagonist of the ergoline and lysergamide families related to methysergide which was under development by Eli Lilly and Company for the treatment of a variety of conditions including depression, anxiety, schizophrenia, male sexual dysfunction, migraine, and thrombosis but was never marketed. It reached phase II clinical trials for the treatment of depression, erectile dysfunction, and premature ejaculation prior to the discontinuation of its development.

The Watering Trough at Marly with Hoarfrost is an 1876 painting by Alfred Sisley. It was owned by François Depeaux, a Sisley collector, and passed through other collections before ending up in that of Paul Mellon. It is now in the Virginia Museum of Fine Arts in Richmond, United States. It was painted at Marly-le-Roi and is part of his Marly series. Sisley's works showing the watering trough and The Flood at Port-Marly are two series of Impressionist masterworks comparable to Claude Monet's Gare Lazare series, Renoir's The Swing and Bal du moulin de la Galette series, Berthe Morisot's Champs de blé series and Camille Pissarro's Vues de Pontoise and Toits rouges series. Sisley did not much change his point of view between each painting, but he dramatically changed the background, proving his ability to vary views of a limited section of countryside.















