 | |
| Names | |
|---|---|
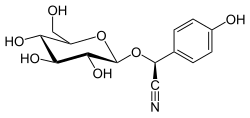
| IUPAC name (S)-(β-D-Glucopyranosyloxy)(4-hydroxyphenyl)acetonitrile | |
| Systematic IUPAC name (S)-(4-Hydroxyphenyl){[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}acetonitrile | |
| Other names (S)-4-Hydroxymandelnitrile-β-D-glucopyranoside | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.007.163 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C14H17NO7 | |
| Molar mass | 311.29 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
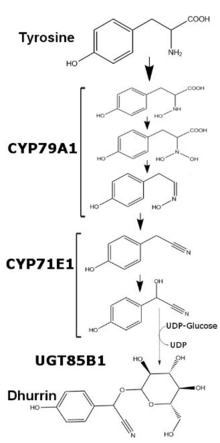
Dhurrin is a cyanogenic glycoside produced in many plants. Discovered in multiple sorghum varieties in 1906 as the culprit of cattle poisoning by hydrogen cyanide, dhurrin is most typically associated with Sorghum bicolor , [1] the organism used for mapping the biosynthesis of dhurrin from tyrosine. Dhurrin's name is derived from the Arabic word for sorghum.