Related Research Articles

Venom or zootoxin is a type of toxin produced by an animal that is actively delivered through a wound by means of a bite, sting, or similar action. The toxin is delivered through a specially evolved venom apparatus, such as fangs or a stinger, in a process called envenomation. Venom is often distinguished from poison, which is a toxin that is passively delivered by being ingested, inhaled, or absorbed through the skin, and toxungen, which is actively transferred to the external surface of another animal via a physical delivery mechanism.

Batrachotoxin (BTX) is an extremely potent cardio- and neurotoxic steroidal alkaloid found in certain species of beetles, birds, and frogs. The name is from the Greek word βάτραχος, bátrachos, 'frog'. Structurally-related chemical compounds are often referred to collectively as batrachotoxins. In certain frogs, this alkaloid is present mostly on the skin. Such frogs are among those used for poisoning darts. Batrachotoxin binds to and irreversibly opens the sodium channels of nerve cells and prevents them from closing, resulting in paralysis and death. No antidote is known.

Palytoxin, PTX or PLTX is an intense vasoconstrictor, and is considered to be one of the most poisonous non-protein substances known, second only to maitotoxin in terms of toxicity in mice.

Cardiotoxin III is a sixty amino-acid polypeptide toxin from the Taiwan Cobra Naja atra. CTX III is highly basic and hydrophobic protein. It is an example of a group of snake cardio/cytotoxins, which are made up of shorter snake venom three-finger toxins. Over 50 different cytotoxin polypeptides have been isolated and sequenced from venom samples. The difference in the CTX functionality may be due to the relatively small difference in the polypeptide's structure, allowing different CTXs to induce lysis in different cell types. The CTX III molecule contains multiple binding sites and is cytolytic for myocardial cells and human leukemic T cells.
A latrotoxin is a high-molecular mass neurotoxin found in the venom of spiders of the genus Latrodectus as well as at least one species of another genus in the same family, Steatoda nobilis. Latrotoxins are the main active components of the venom and are responsible for the symptoms of latrodectism.
Taicatoxin (TCX) is a snake toxin that blocks voltage-dependent L-type calcium channels and small conductance Ca2+-activated K+ channels. The name taicatoxin (TAIpan + CAlcium + TOXIN) is derived from its natural source, the taipan snake, the site of its action, calcium channels, and from its function as a toxin. Taicatoxin was isolated from the venom of Australian taipan snake, Oxyuranus scutellatus scutellatus. TCX is a secreted protein, produced in the venom gland of the snake.
Tityustoxin is a toxin found in the venom of scorpions from the subfamily Tityinae. By binding to voltage-dependent sodium ion channels and potassium channels, they cause sialorrhea, lacrimation and rhinorrhea.
Taipoxin is a potent myo- and neurotoxin that was isolated from the venom of the coastal taipan Oxyuranus scutellatus or also known as the common taipan. Taipoxin like many other pre-synaptic neurotoxins are phospholipase A2 (PLA2) toxins, which inhibit/complete block the release of the motor transmitter acetylcholine and lead to death by paralysis of the respiratory muscles (asphyxia). It is the most lethal neurotoxin isolated from any snake venom to date.

Pardachirus marmoratus, the finless sole, speckled sole or Red Sea Moses sole, is a species of flatfish native to the western Indian Ocean.
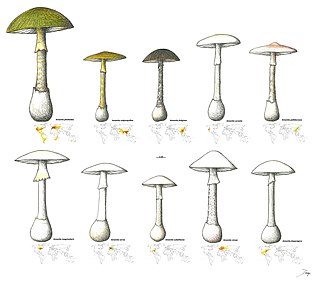
Phallolysin is a protein found the Amanita phalloides species of the Amanita genus of mushrooms, the species commonly known as the death cap mushroom. The protein is toxic and causes cytolysis in many cells found in animals and is noted for its hemolytic properties. It was one of the first toxins discovered in Amanita phalloides when the various toxins in the species where first being researched. The protein itself is observed to come in 3 variations, with observed differences in isoelectric point. Cytolysis can be best described as being the destruction of cells, likely due to exposure from an external source such as pathogens and toxins. Hemolysis then follows a similar destructive pathway, but instead focuses specifically on the destruction of red blood cells. Phallolysin is known to be thermolabile, meaning that it is destroyed at high temperatures, and acid labile, meaning that it is easily broken down in acidic environments.

Pumiliotoxin 251D is a toxic organic compound. It is found in the skin of poison frogs from the genera Dendrobates, Epipedobates, Minyobates, and Phyllobates and toads from the genus Melanophryniscus. Its name comes from the pumiliotoxin family (PTXs) and its molecular mass of 251 daltons. When the toxin enters the bloodstream through cuts in the skin or by ingestion, it can cause hyperactivity, convulsions, cardiac arrest and ultimately death. It is especially toxic to arthropods, even at low concentrations.

Diamphidia, or Bushman arrow-poison beetle, is an African genus of flea beetles, in the family Chrysomelidae.

The high-affinity choline transporter (ChT) also known as solute carrier family 5 member 7 is a protein in humans that is encoded by the SLC5A7 gene. It is a cell membrane transporter and carries choline into acetylcholine-synthesizing neurons.
Bestoxin is a neurotoxin from the venom of the South African spitting scorpion Parabuthus transvaalicus. Most likely, it targets sodium channel function, thus promoting spontaneous and repetitive neuronal firing. Following injection into mice, it causes non-lethal writhing behaviour.

Diamphidia nigroornata or Bushman arrow-poison beetle, is an African leaf beetle species in the genus Diamphidia.

Toxin Cll1 is a toxin from the venom of the Mexican scorpion Centruroides limpidus limpidus, which changes the activation threshold of sodium channels by binding to neurotoxin binding site 4, resulting in increased excitability.
Grammistins are peptide toxins synthesised by glands in the skin of soapfishes of the tribes Grammistini and Diploprionini which are both classified within the grouper subfamily Epinephelinae, a part of the family Serranidae. Grammistin has a hemolytic and ichthyotoxic action. The grammistins have secondary structures and biological effects comparable to other classes of peptide toxins, melittin from the bee stings and pardaxins which are secreted in the skin of two sole species. A similar toxin has been found to be secreted in the skin of some clingfishes.
Phoratoxins are a group of peptide toxins that belong to the family of thionins, a subdivision of small plant toxins. Phoratoxins are proteins present in the leaves and branches of the Phoradendron, commonly known as the American variant of the mistletoe, a plant commonly used as decoration during the festive season. The berries of the mistletoe do not contain phoratoxins, making them less toxic compared to other parts of the plant. The toxicity of the mistletoe is dependent on the host tree, since mistletoe is known to be a semi-parasite. The host tree provides fixed inorganic nitrogen compounds necessary for the mistletoe to synthesize phoratoxins.
U7-ctenitoxin-Pn1a (or U7-CNTX-Pn1a for short) is a neurotoxin that blocks TRPV1 channels, and can exhibit analgestic effects. It is naturally found in the venom of Phoneutria nigriventer.
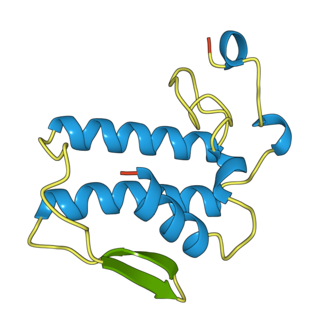
Notexin is a toxin produced by the tiger snake (Notechis scutatus). It is a myotoxic and presynaptic, neurotoxic phospholipase A2 (PLA2s). These are enzymes that hydrolyze the bond between a fatty acid tail and glycerol in fatty acids on the 2-position.
References
- 1 2 de la Harpe, J.; Reich, E.; Reich, K. A.; Dowdle, E. B. (October 1983). "Diamphotoxin. The arrow poison of the !Kung Bushmen". The Journal of Biological Chemistry . 258 (19): 11924–31. doi: 10.1016/S0021-9258(17)44321-3 . hdl: 11427/27499 . PMID 6311829 . Retrieved 4 July 2013.
- ↑ Mebs, D.; Brüning, F.; Pfaff, N.; Neuwinger, H. D. (July 1982). "Preliminary studies on the chemical properties of the toxic principle from Diamphidia nigroornata larvae, a source of Bushman arrow poison". Journal of Ethnopharmacology . 6 (1): 1–11. doi:10.1016/0378-8741(82)90068-X. PMID 7109661.
- ↑ Woollard, J. M.; Fuhrman, F. A.; Mosher, H. S. (1984). "The Bushman arrow toxin, Diamphidia toxin: Isolation from pupae of Diamphidia nigro-ornata". Toxicon . 22 (6): 937–46. doi:10.1016/0041-0101(84)90185-5. PMID 6523515.
- ↑ Jacobsen, T. F.; Sand, O.; Bjøro, T.; Karlsen, H. E.; Iversen, J. G. (1990). "Effect of Diamphidia toxin, a Bushman arrow poison, on ionic permeability in nucleated cells". Toxicon . 28 (4): 435–44. doi:10.1016/0041-0101(90)90082-i. PMID 2161574.
- ↑ Kao, C. Y.; Salwen, M. J.; Hu, S. L.; Pitter, H. M.; Woollard, J. M. (1989). "Diamphidia toxin, the Bushmen's arrow poison: Possible mechanism of prey-killing". Toxicon . 27 (12): 1351–66. doi:10.1016/0041-0101(89)90067-6. PMID 2629177.
- 1 2 Chaboo, Caroline (2011). "Defensive behaviors in leaf beetles: From the unusual to the weird" (PDF). In Weir, Tiffany; Vivanco, Jorge M. (eds.). Chemical Biology of the Tropics: An Interdisciplinary Approach. Signaling and Communication in Plants. Berlin: Springer Verlag. pp. 59–69. ISBN 978-3-642-19079-7. OCLC 706961677. Archived from the original (PDF) on 7 December 2013. Retrieved 4 July 2013.