
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.

Cis–trans isomerism, also known as geometric isomerism or configurational isomerism, is a term used in chemistry that concerns the spatial arrangement of atoms within molecules. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some plane, while trans conveys that they are on opposing (transverse) sides. Cis–trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. Cis-trans notation does not always correspond to E–Z isomerism, which is an absolute stereochemical description. In general, cis–trans stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevented. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "syn" and "anti" are used.
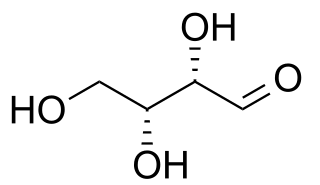
In stereochemistry, diastereomers are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two.
An antifreeze is an additive which lowers the freezing point of a water-based liquid. An antifreeze mixture is used to achieve freezing-point depression for cold environments. Common antifreezes also increase the boiling point of the liquid, allowing higher coolant temperature. However, all common antifreeze additives also have lower heat capacities than water, and do reduce water's ability to act as a coolant when added to it.

A refrigerant is a working fluid used in the refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated due to their toxicity, flammability and the contribution of CFC and HCFC refrigerants to ozone depletion and that of HFC refrigerants to climate change.

Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two are called isopentane (methylbutane) and neopentane (dimethylpropane). Cyclopentane is not an isomer of pentane because it has only 10 hydrogen atoms where pentane has 12.
Demeton-S-methyl is an organic compound with the molecular formula C6H15O3PS2. It was used as an organothiophosphate acaricide and organothiophosphate insecticide. It is flammable. With prolonged storage, Demeton-S-methyl becomes more toxic due to formation of a sulfonium derivative which has greater affinity to the human form of the acetylcholinesterase enzyme, and this may present a hazard in agricultural use.
A coolant is a substance, typically liquid or gas, that is used to reduce or regulate the temperature of a system. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, chemically inert and neither causes nor promotes corrosion of the cooling system. Some applications also require the coolant to be an electrical insulator.
But-2-ene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism ; that is, it exists as two geometric isomers cis-but-2-ene ( -but-2-ene) and trans-but-2-ene ( -but-2-ene).
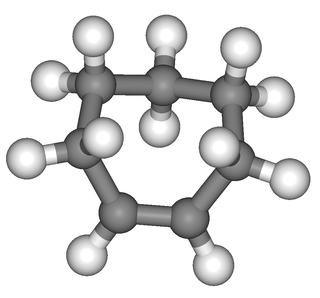
Cycloheptene is a 7-membered cycloalkene with a flash point of −6.7 °C. It is a raw material in organic chemistry and a monomer in polymer synthesis. Cycloheptene can exist as either the cis- or the trans-isomer.
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula C8H12, specifically [−(CH2)2−CH=CH−]2.
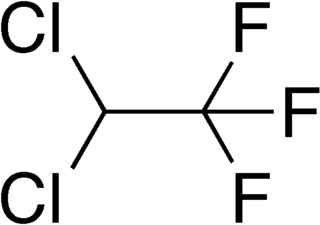
2,2-Dichloro-1,1,1-trifluoroethane or HCFC-123 is considered as an alternative to CFC-11 in low pressure refrigeration and HVAC systems, and should not be used in foam blowing processes or solvent applications.
Xylidine can refer to any of the six isomers of xylene amine, or any mixture of them.
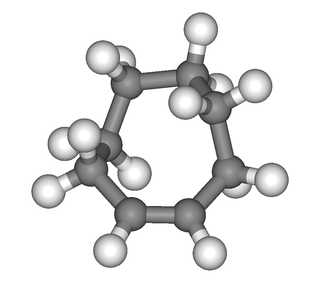
trans-Cyclooctene is a cyclic hydrocarbon with the formula [–(CH2)6CH=CH–], where the two C–C single bonds adjacent to the double bond are on opposite sides of the latter's plane. It is a colorless liquid with a disagreeable odor.

1,1-Dichloro-1-fluoroethane is a haloalkane with the formula C
2H
3Cl
2F. It is one of the three isomers of dichlorofluoroethane. It belongs to the hydrochlorofluorocarbon (HCFC) family of man-made compounds that contribute significantly to both ozone depletion and global warming when released into the environment.

Cyclohexanedimethanol (CHDM) is a mixture of isomeric organic compounds with formula C6H10(CH2OH)2. It is a colorless low-melting solid used in the production of polyester resins. Commercial samples consist of a mixture of cis and trans isomers. It is a di-substituted derivative of cyclohexane and is classified as a diol, meaning that it has two OH functional groups. Commercial CHDM typically has a cis/trans ratio of 30:70.

1,3,3,3-Tetrafluoropropene (HFO-1234ze(E), R-1234ze) is a hydrofluoroolefin. It was developed as a "fourth generation" refrigerant to replace fluids such as R-134a, as a blowing agent for foam and aerosol applications, and in air horns and gas dusters. The use of R-134a is being phased out because of its high global warming potential (GFA). HFO-1234ze(E) itself has zero ozone-depletion potential (ODP=0), a very low global warming potential (GWP < 1 ), even lower than CO2, and it is classified by ANSI/ASHRAE as class A2L refrigerant (lower flammability and lower toxicity). In open atmosphere however, HFO-1234ze actually might form HFC-23 as one of its secondary atmospheric breakdown products. HFC-23 is a very potent greenhouse gas with a GWP100 of 14,800. The secondary GWP of R-1234ze would then be in the range of 1,400±700 considering the amount of HFC-23 which may form from HFO-1234ze in the atmosphere. Besides the global warming potential, when HFOs decompose in the atmosphere, trifluoroacetic acid (TFA(A)) is formed, which also remains in the atmosphere for several days. The trifluoroacetic acid then forms trifluoroacetate (TFA), a salt of trifluoroacetic acid, in water and on the ground. Due to its high polarity and low degradability, it is difficult to remove TFA from drinking water (ICPR 2019).

4-Methylcyclohexanemethanol (MCHM, systematic name 4-methylcyclohexylmethanol) is an organic compound with the formula CH3C6H10CH2OH. Classified as a saturated higher alicyclic primary alcohol. Both cis and trans isomers exist, depending on the relative positions of the methyl (CH3) and hydroxymethyl (CH2OH) groups on the cyclohexane ring. Commercial samples of MCHM consists of a mixture of these isomers as well as other components that vary with the supplier.
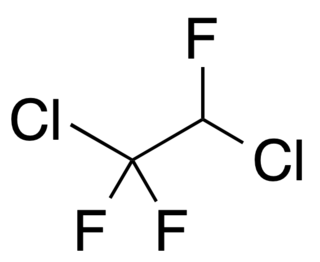
1,2-Dichloro-1,1,2-trifluoroethane is a volatile liquid chlorofluoroalkane composed of carbon, hydrogen, chlorine and fluorine, and with structural formula CClF2CHClF. It is also known as a refrigerant with the designation R-123a.















