Related Research Articles

Agarose is a heteropolysaccharide, generally extracted from certain red seaweed. It is a linear polymer made up of the repeating unit of agarobiose, which is a disaccharide made up of D-galactose and 3,6-anhydro-L-galactopyranose. Agarose is one of the two principal components of agar, and is purified from agar by removing agar's other component, agaropectin.

Herbicides, also commonly known as weed killers, are substances used to control undesired plants, also known as weeds. Selective herbicides control specific weed species while leaving the desired crop relatively unharmed, while non-selective herbicides kill plants indiscriminately. The combined effects of herbicides, nitrogen fertilizer, and improved cultivars has increased yields of major crops by 3x to 6x from 1900 to 2000.

Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecules pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid, which crystallizes in needles. There are few commercial applications of acridines; at one time acridine dyes were popular, but they are now relegated to niche applications, such as with acridine orange. The name is a reference to the acrid odour and acrid skin-irritating effect of the compound.

BASF SE, an initialism of its original name Badische Anilin- und Sodafabrik, is a European multinational company and the largest chemical producer in the world. Its headquarters are located in Ludwigshafen, Germany.

Clopyralid is a selective herbicide used for control of broadleaf weeds, especially thistles and clovers. Clopyralid is in the picolinic acid family of herbicides, which also includes aminopyralid, picloram, triclopyr, and several less common herbicides. For control of creeping thistle, Cirsium arvense, a noxious, perennial weed, clopyralid is one of the few effective herbicides available. It is particularly damaging to peas, tomatoes, and sunflowers, and can render potatoes, lettuce, and spinach inedible. It does not affect grasses.

Arecoline is a nicotinic acid-based mild parasympathomimetic stimulant alkaloid found in the areca nut, the fruit of the areca palm. It is an odourless oily liquid. It can bring a sense of enhanced alertness and energy along with mild feelings of euphoria and relaxation. The psychoactive effects are comparable to that of nicotine.

Phenoxy herbicides are two families of chemicals that have been developed as commercially important herbicides, widely used in agriculture. They share the part structure of phenoxyacetic acid.

Nicomorphine is the 3,6-dinicotinate ester of morphine. It is a strong opioid agonist analgesic two to three times as potent as morphine with a side effect profile similar to that of dihydromorphine, morphine, and diamorphine.
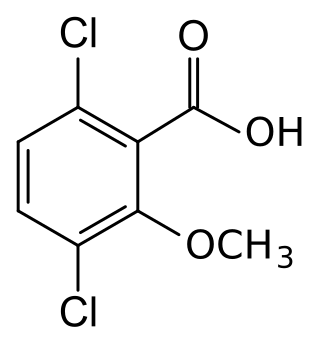
Dicamba is a selective systemic herbicide first registered in 1967. Brand names for formulations of this herbicide include Dianat, Banvel, Diablo, Oracle and Vanquish. This chemical compound is a chlorinated derivative of o-anisic acid. It has been described as a "widely used, low-cost, environmentally friendly herbicide that does not persist in soils and shows little or no toxicity to wildlife and humans."

Mecoprop is a common general use herbicide found in many household weed killers and "weed-and-feed" type lawn fertilizers. It is primarily used to control broadleaf weeds. It is often used in combination with other chemically related herbicides such as 2,4-D, dicamba, and MCPA, which mimic the plant hormone IAA (auxin) and kill most broadleaf weeds by causing uncontrolled growth.

Cytochrome c oxidase subunit 7B, mitochondrial (COX7B) is an enzyme that in humans is encoded by the COX7B gene. COX7B is a nuclear-encoded subunit of cytochrome c oxidase (COX). Cytochrome c oxidase is a multi-subunit enzyme complex that couples the transfer of electrons from cytochrome c to molecular oxygen and contributes to a proton electrochemical gradient across the inner mitochondrial membrane, acting as the terminal enzyme of the mitochondrial respiratory chain. Work with Oryzias latices has linked disruptions in COX7B with microphthalmia with linear skin lesions (MLS), microcephaly, and mitochondrial disease. Clinically, mutations in COX7B have been associated with linear skin defects with multiple congenital anomalies.

In computing, ren is a command in various command-line interpreters (shells) such as COMMAND.COM, cmd.exe, 4DOS, 4NT and Windows PowerShell. It is used to rename computer files and in some implementations also directories. It is analogous to the Unix mv command. However, unlike mv, ren cannot be used to move files, as a new directory for the destination file may not be used. Alternatively, move may be used if available. On versions of MS-DOS that do not support the move command, the user would simply copy the file to a new destination, and then delete the original file. A notable exception to this rule is DOSBox, in which ren may be used to move a file, since move is not supported.

Sodium chromate is the inorganic compound with the formula Na2CrO4. It exists as a yellow hygroscopic solid, which can form tetra-, hexa-, and decahydrates. It is an intermediate in the extraction of chromium from its ores.
The molecular formula C8H6Cl2O3 (molar mass: 221.03 g/mol) may refer to:
The molecular formula C6H4O4 may refer to:
Diaminohexanoic acid may refer to:
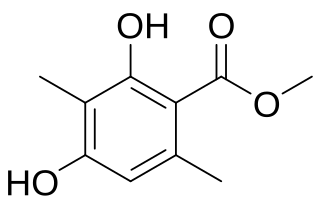
Atraric acid is a naturally occurring phenolic compound and ester with the IUPAC name methyl 2,4-dihydroxy-3,6-dimethylbenzoate and molecular formula C10H12O4. It occurs in the root-bark of Pygeum africanum and Evernia prunastri (Oakmoss). There is evidence to suggest that it has antiandrogenic activity in humans and its use in treatment of benign prostate hyperplasia, prostate cancer, and spinal and bulbar muscular atrophy has been investigated.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.

Barbatic acid is an organic compound that is made by some lichens. It is in the structural class known as depsides. It is particularly common in the genera Usnea and Cladonia.
Chlorosalicylic acid may refer to:
References
- ↑ Dumitru, Razvan; Jiang, Wen Zhi; Weeks, Donald P.; Wilson, Mark A. (2009). "Crystal Structure of Dicamba Monooxygenase: A Rieske Nonheme Oxygenase that Catalyzes Oxidative Demethylation". Journal of Molecular Biology. 392 (2): 498–510. doi:10.1016/j.jmb.2009.07.021. PMC 3109874 . PMID 19616011.