This article needs additional citations for verification .(December 2024) |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 2,2′-Bioxirane | |
| Other names 1,1′-Bi[ethylene oxide]; 1,2:3,4-Diepoxybutane; 1,3-Butadiene diepoxide; Bioxirane; Butadiene dioxide; Butane diepoxide; Dioxybutadiene | |
| Identifiers | |
| |
3D model (JSmol) | |
| Abbreviations | DEB |
| 79831 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.014.527 |
| EC Number |
|
PubChem CID | |
| UNII |
|
| UN number | 3384 3082 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C4H6O2 | |
| Molar mass | 86.090 g·mol−1 |
| Density | 1.113 g/cm3 (18 °C) [1] |
| Melting point | 4 °C (39 °F; 277 K) [1] |
| Boiling point | 138 °C (280 °F; 411 K) [1] |
| Miscible [1] | |
| Vapor pressure | 0.52 kPa (at 20 °C) [2] |
| Hazards | |
| GHS labelling: | |
    | |
| Danger | |
| H226, H301, H310, H311, H314, H330, H340, H350 | |
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P262, P264, P270, P271, P280, P281, P284, P301+P310, P301+P330+P331, P302+P350, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P312, P320, P321, P322, P330, P361, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Flash point | 46 °C (115 °F; 319 K) [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
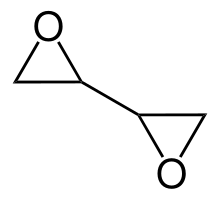
Diepoxybutane (also known as butane diepoxide, butadiene diepoxide, or 1,2:3,4-diepoxybutane) is an epoxide which is a colorless liquid at room temperature. Epoxides are very reactive due to ring strain and diepoxybutane contains two of these groups, so it is highly reactive, more than other ethers.[ which? ] It is hydrophilic, very flammable and easily ignited by heat or sparks.[ citation needed ]
Contents
- Structure, reactivity, synthesis
- Other uses
- Toxicity
- Effect on humans
- Carcinogenicity
- Effect on animals
- References
Diepoxybutane is used as a chemical intermediate, as a cross-linking agent for polymers and textiles, and as a preservative.[ clarification needed ] [2] [ better source needed ]