
A centrifuge is a device that uses centrifugal force to subject a specimen to a specified constant force - for example, to separate various components of a fluid. This is achieved by spinning the fluid at high speed within a container, thereby separating fluids of different densities or liquids from solids. It works by causing denser substances and particles to move outward in the radial direction. At the same time, objects that are less dense are displaced and moved to the centre. In a laboratory centrifuge that uses sample tubes, the radial acceleration causes denser particles to settle to the bottom of the tube, while low-density substances rise to the top. A centrifuge can be a very effective filter that separates contaminants from the main body of fluid.

An ultracentrifuge is a centrifuge optimized for spinning a rotor at very high speeds, capable of generating acceleration as high as 1 000 000 g. There are two kinds of ultracentrifuges, the preparative and the analytical ultracentrifuge. Both classes of instruments find important uses in molecular biology, biochemistry, and polymer science.

In chemistry, a Svedberg unit or svedberg is a non-SI metric unit for sedimentation coefficients. The Svedberg unit offers a measure of a particle's size indirectly based on its sedimentation rate under acceleration. The svedberg is a measure of time, defined as exactly 10−13 seconds (100 fs).

Decantation is a process for the separation of mixtures of immiscible liquids or of a liquid and a solid mixture such as a suspension. The layer closer to the top of the container—the less dense of the two liquids, or the liquid from which the precipitate or sediment has settled out—is poured off, leaving denser liquid or the solid behind. The process typically is unable to remove all of the top layer, meaning the separation is incomplete or at least one of the two separated components is still contaminated by the other one.

Centrifugation is a mechanical process which involves the use of the centrifugal force to separate particles from a solution according to their size, shape, density, medium viscosity and rotor speed. The denser components of the mixture migrate away from the axis of the centrifuge, while the less dense components of the mixture migrate towards the axis. Chemists and biologists may increase the effective gravitational force of the test tube so that the precipitate (pellet) will travel quickly and fully to the bottom of the tube. The remaining liquid that lies above the precipitate is called a supernatant or supernate.
In cell biology, cell fractionation is the process used to separate cellular components while preserving individual functions of each component. This is a method that was originally used to demonstrate the cellular location of various biochemical processes. Other uses of subcellular fractionation is to provide an enriched source of a protein for further purification, and facilitate the diagnosis of various disease states.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the specification of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Ideally, to study a protein of interest, it must be separated from other components of the cell so that contaminants will not interfere in the examination of the protein of interest's structure and function. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.

A synaptosome is an isolated synaptic terminal from a neuron. Synaptosomes are obtained by mild homogenization of nervous tissue under isotonic conditions and subsequent fractionation using differential and density gradient centrifugation. Liquid shear detaches the nerve terminals from the axon and the plasma membrane surrounding the nerve terminal particle reseals. Synaptosomes are osmotically sensitive, contain numerous small clear synaptic vesicles, sometimes larger dense-core vesicles and frequently one or more small mitochondria. They carry the morphological features and most of the chemical properties of the original nerve terminal. Synaptosomes isolated from mammalian brain often retain a piece of the attached postsynaptic membrane, facing the active zone.

Analytical ultracentrifugation is an analytical technique which combines an ultracentrifuge with optical monitoring systems.
In chemistry, the sedimentation coefficient of a particle characterizes its sedimentation during centrifugation. It is defined as the ratio of a particle's sedimentation velocity to the applied acceleration causing the sedimentation.

Elutriation is a process for separating particles based on their size, shape and density, using a stream of gas or liquid flowing in a direction usually opposite to the direction of sedimentation. This method is mainly used for particles smaller than 1 μm. The smaller or lighter particles rise to the top (overflow) because their terminal sedimentation velocities are lower than the velocity of the rising fluid. The terminal velocity of any particle in any medium can be calculated using Stokes' law if the particle's Reynolds number is below 0.2. Counterflow centrifugation elutriation is a related technique to separate cells.

A laboratory centrifuge is a piece of laboratory equipment, driven by a motor, which spins liquid samples at high speed. There are various types of centrifuges, depending on the size and the sample capacity.
Sedimentation potential occurs when dispersed particles move under the influence of either gravity or centrifugation or electricity in a medium. This motion disrupts the equilibrium symmetry of the particle's double layer. While the particle moves, the ions in the electric double layer lag behind due to the liquid flow. This causes a slight displacement between the surface charge and the electric charge of the diffuse layer. As a result, the moving particle creates a dipole moment. The sum of all of the dipoles generates an electric field which is called sedimentation potential. It can be measured with an open electrical circuit, which is also called sedimentation current.

Field-flow fractionation, abbreviated FFF, is a separation technique invented by J. Calvin Giddings. The technique is based on separation of colloidal or high molecular weight substances in liquid solutions, flowing through the separation platform, which does not have a stationary phase. It is similar to liquid chromatography, as it works on dilute solutions or suspensions of the solute, carried by a flowing eluent. Separation is achieved by applying a field or cross-flow, perpendicular to the direction of transport of the sample, which is pumped through a long and narrow laminar channel. The field exerts a force on the sample components, concentrating them towards one of the channel walls, which is called accumulation wall. The force interacts with a property of the sample, thereby the separation occurs, in other words, the components show differing "mobilities" under the force exerted by the crossing field. As an example, for the hydraulic, or cross-flow FFF method, the property driving separation is the translational diffusion coefficient or the hydrodynamic size. For a thermal field, it is the ratio of the thermal and the translational diffusion coefficient.
Counterflow centrifugal elutriation (CCE) is a liquid clarification technique. This method enables scientists to separate different cells with different sizes. Since cell size is correlated with cell cycle stages this method also allows the separation of cells at different stages of the cell cycle.
The peeler centrifuge is a device that performs by rotating filtration basket in an axis. A centrifuge follows on the principle of centrifugal force to separate solids from liquids by density difference. High rotation speed provides high centrifugal force that allows the suspended solid in feed to settle on the inner surface of basket. There are three kinds of centrifuge, horizontal, vertical peeler centrifuge and siphon peeler centrifuge. These classes of instrument apply to various areas such as fertilisers, pharmaceutical, plastics and food including artificial sweetener and modified starch.
A centrifuge is a device that employs a high rotational speed to separate components of different densities. This becomes relevant in the majority of industrial jobs where solids, liquids and gases are merged into a single mixture and the separation of these different phases is necessary. A decanter centrifuge separates continuously solid materials from liquids in the slurry, and therefore plays an important role in the wastewater treatment, chemical, oil, and food processing industries. There are several factors that affect the performance of a decanter centrifuge, and some design heuristics are to be followed which are dependent upon given applications.

A solid bowl centrifuge is a type of centrifuge that uses the principle of sedimentation. A centrifuge is used to separate a mixture that consists of two substances with different densities by using the centrifugal force resulting from continuous rotation. It is normally used to separate solid-liquid, liquid-liquid, and solid-solid mixtures. Solid bowl centrifuges are widely used in various industrial applications, such as wastewater treatment, coal manufacturing, and polymer manufacturing. One advantage of solid bowl centrifuges for industrial uses is the simplicity of installation compared to other types of centrifuge. There are three design types of solid bowl centrifuge, which are conical, cylindrical, and conical-cylindrical.
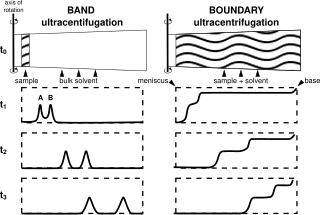
Analytical band centrifugation (ABC) (also known as analytical band ultracentrifugation, or band sedimentation-velocity), is a specialized ultracentrifugation procedure, where unlike the typical use of (boundary) sedimentation velocity analytical ultracentrifugation (SV-AUC) wherein a homogenous bulk solution is centrifuged, in ABC a thin (~15 μL, ~500 μm) sample is layered on top of a bulk solvent and then centrifuged. The method is distinguished from zone-sedimentation in that a stabilizing density gradient is self-generated during centrifugation, through the use of a higher density (than the sample) bulk "binary solvent", containing both a solvent (i.e. H2O), and a second component (small molecules, i.e. CsCl) that will sediment to form a stabilizing density gradient for the sample.
A centrifuge is a device that uses centrifugal force to separate its components.












