
Propranolol, sold under the brand name Inderal among others, is a medication of the beta blocker class. It is used to treat high blood pressure, a number of types of irregular heart rate, thyrotoxicosis, capillary hemangiomas, performance anxiety, and essential tremors, as well to prevent migraine headaches, and to prevent further heart problems in those with angina or previous heart attacks. It can be taken by mouth or by injection into a vein. The formulation that is taken by mouth comes in short-acting and long-acting versions. Propranolol appears in the blood after 30 minutes and has a maximum effect between 60 and 90 minutes when taken by mouth.

The adrenergic receptors or adrenoceptors are a class of G protein-coupled receptors that are targets of many catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) produced by the body, but also many medications like beta blockers, beta-2 (β2) agonists and alpha-2 (α2) agonists, which are used to treat high blood pressure and asthma, for example.
Agmatine, also known as (4-aminobutyl-guanidine) is an aminoguanidine that was discovered in 1910 by Albrecht Kossel. Agmatine is a chemical substance which is naturally created from the amino acid arginine. Agmatine has been shown to exert modulatory action at multiple molecular targets, notably: neurotransmitter systems, ion channels, nitric oxide (NO) synthesis and polyamine metabolism and this provides bases for further research into potential applications.
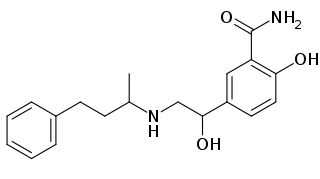
Labetalol is a medication used to treat high blood pressure and in long term management of angina. This includes essential hypertension, hypertensive emergencies, and hypertension of pregnancy. In essential hypertension it is generally less preferred than a number of other blood pressure medications. It can be given by mouth or by injection into a vein.
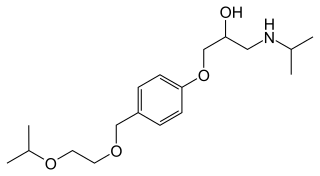
Bisoprolol, sold under the brand name Zebeta among others, is a beta blocker medication used for heart diseases. This includes high blood pressure, chest pain from not enough blood flow to the heart, and heart failure. It is taken by mouth.

Penbutolol is a medication in the class of beta blockers, used in the treatment of high blood pressure. Penbutolol is able to bind to both beta-1 adrenergic receptors and beta-2 adrenergic receptors, thus making it a non-selective β blocker. Penbutolol is a sympathomimetic drug with properties allowing it to act as a partial agonist at β adrenergic receptors.
alpha-1 (α1) adrenergic receptors are G protein-coupled receptors (GPCRs) associated with the Gq heterotrimeric G protein. α1-adrenergic receptors are subdivided into three highly homologous subtypes, i.e., α1A-, α1B-, and α1D-adrenergic receptor subtypes. There is no α1C receptor. At one time, there was a subtype known as α1C, but it was found to be identical to the previously discovered α1A receptor subtype. To avoid confusion, naming was continued with the letter D. Catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) signal through the α1-adrenergic receptors in the central and peripheral nervous systems. The crystal structure of the α1B-adrenergic receptor subtype has been determined in complex with the inverse agonist (+)-cyclazosin.
The alpha-2 (α2) adrenergic receptor is a G protein-coupled receptor (GPCR) associated with the Gi heterotrimeric G-protein. It consists of three highly homologous subtypes, including α2A-, α2B-, and α2C-adrenergic. Some species other than humans express a fourth α2D-adrenergic receptor as well. Catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) signal through the α2-adrenergic receptor in the central and peripheral nervous systems.

The beta-1 adrenergic receptor, also known as ADRB1, is a beta-adrenergic receptor, and also denotes the human gene encoding it. It is a G-protein coupled receptor associated with the Gs heterotrimeric G-protein and is expressed predominantly in cardiac tissue.
The beta-2 adrenergic receptor, also known as ADRB2, is a cell membrane-spanning beta-adrenergic receptor that binds epinephrine (adrenaline), a hormone and neurotransmitter whose signaling, via adenylate cyclase stimulation through trimeric Gs proteins, increased cAMP, and downstream L-type calcium channel interaction, mediates physiologic responses such as smooth muscle relaxation and bronchodilation.
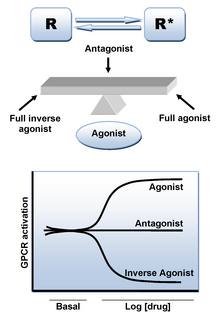
An adrenergic antagonist is a drug that inhibits the function of adrenergic receptors. There are five adrenergic receptors, which are divided into two groups. The first group of receptors are the beta (β) adrenergic receptors. There are β1, β2, and β3 receptors. The second group contains the alpha (α) adrenoreceptors. There are only α1 and α2 receptors. Adrenergic receptors are located near the heart, kidneys, lungs, and gastrointestinal tract. There are also α-adreno receptors that are located on vascular smooth muscle.

The alpha-2A adrenergic receptor, also known as ADRA2A, is an α2 adrenergic receptor, and also denotes the human gene encoding it.

The beta-3 adrenergic receptor (β3-adrenoceptor), also known as ADRB3, is a beta-adrenergic receptor, and also denotes the human gene encoding it.

Denopamine (INN) is a cardiotonic drug which acts as a β1 adrenergic receptor agonist. It is used in the treatment of angina and may also have potential uses in the treatment of congestive heart failure and for clearing pulmonary oedema. It is marketed in Japan under the brand name Kalgut (カルグート) and available as tablets of 5 and 10 mg, and 5% fine granules.
Beta1-adrenergic agonists, also known as Beta1-adrenergic receptor agonists, are a class of drugs that bind selectively to the beta-1 adrenergic receptor. As a result, they act more selectively upon the heart. Beta-adrenoceptors typically bind to norepinephrine release by sympathetic adrenergic nerves and to circulating epinephrine. The effect of B-adrenoceptors is cardiac stimulation, such as increased heart rate, heart contractility, heart conduction velocity and heart relaxation.

ICI-118,551 is a selective β2 adrenergic receptor (adrenoreceptor) antagonist or beta blocker. ICI binds to the β2 subtype with at least 100 times greater affinity than β1 or β3, the two other known subtypes of the beta adrenoceptor. The compound was developed by Imperial Chemical Industries, which was acquired by AkzoNobel in 2008.

Dextrallorphan (DXA) is an chemical of the morphinan class that is used in scientific research. It acts as a σ1 receptor agonist and NMDA receptor antagonist. It has no significant affinity for the σ2, μ-opioid, or δ-opioid receptor, or for the serotonin or norepinephrine transporter. As an NMDA receptor antagonist, in vivo, it is approximately twice as potent as dextromethorphan, and five-fold less potent than dextrorphan.
β2-adrenoceptor agonists is a group of drugs that act selectively on β2-receptors in the lungs causing bronchodilation. β2-agonists are used to treat asthma and COPD, diseases that cause obstruction in the airways. Prior to their discovery, the non-selective beta-agonist isoprenaline was used. The aim of the drug development through the years has been to minimise side effects, achieve selectivity and longer duration of action. The mechanism of action is well understood and has facilitated the development. The structure of the binding site and the nature of the binding is also well known, as is the structure activity relationship.
The β3 adrenergic receptor agonist or β3-adrenoceptor agonist, also known as β3-AR agonist, are a class of medicine that bind selectively to β3-adrenergic receptors.
Benjamin Weiss is an American neuropharmacologist, Emeritus Professor of Pharmacology and Physiology at Drexel University College of Medicine. He is best known for his work with cyclic nucleotide phosphodiesterases. He was the first to propose, based on his experimental work, that selective inhibition of phosphodiesterases which are expressed differentially in all tissues, could be used as a target for drug development. His work is the basis for many marketed and developmental human drugs that selectively inhibit cyclic nucleotide phosphodiesterases.












