
Bleomycin is a medication used to treat cancer. This includes Hodgkin's lymphoma, non-Hodgkin's lymphoma, testicular cancer, ovarian cancer, and cervical cancer among others. Typically used with other cancer medications, it can be given intravenously, by injection into a muscle or under the skin. It may also be administered inside the chest to help prevent the recurrence of a fluid around the lung due to cancer; however talc is better for this.
Polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (or reduced forms of a ketone) and methylene groups: (-CO-CH2-). First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or better bioactivity.
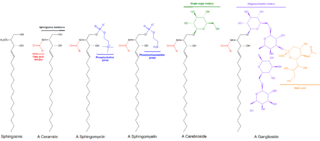
Sphingolipids are a class of lipids containing a backbone of sphingoid bases, a set of aliphatic amino alcohols that includes sphingosine. They were discovered in brain extracts in the 1870s and were named after the mythological sphinx because of their enigmatic nature. These compounds play important roles in signal transduction and cell recognition. Sphingolipidoses, or disorders of sphingolipid metabolism, have particular impact on neural tissue. A sphingolipid with an R group consisting of a hydrogen atom only is a ceramide. Other common R groups include phosphocholine, yielding a sphingomyelin, and various sugar monomers or dimers, yielding cerebrosides and globosides, respectively. Cerebrosides and globosides are collectively known as glycosphingolipids.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.

Ceramides are a family of waxy lipid molecules. A ceramide is composed of sphingosine and a fatty acid. Ceramides are found in high concentrations within the cell membrane of eukaryotic cells, since they are component lipids that make up sphingomyelin, one of the major lipids in the lipid bilayer. Contrary to previous assumptions that ceramides and other sphingolipids found in cell membrane were purely supporting structural elements, ceramide can participate in a variety of cellular signaling: examples include regulating differentiation, proliferation, and programmed cell death (PCD) of cells.

Neocarzinostatin (NCS) is a macromolecular chromoprotein enediyne antitumor antibiotic secreted by Streptomyces macromomyceticus.
Polyketide synthases (PKSs) are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages. The biosyntheses of polyketides share striking similarities with fatty acid biosynthesis.
In enzymology, an erythronolide synthase is an enzyme that catalyzes the chemical reaction

Psymberin, also known as irciniastatin A, is a cytotoxin derived from sea sponges. It was discovered by two independent research groups, one led by Dr. Phil Crews and one led by Dr. Jean Schmidt, in 2004. Psymberin was found to be highly bioactive as it showed LC50s at nanomolar concentrations against various types of tumors.
Streptogramin A is a group of antibiotics within the larger family of antibiotics known as streptogramins. They are synthesized by the bacteria Streptomyces virginiae. The streptogramin family of antibiotics consists of two distinct groups: group A antibiotics contain a 23-membered unsaturated ring with lactone and peptide bonds while group B antibiotics are depsipeptides. While structurally different, these two groups of antibiotics act synergistically, providing greater antibiotic activity than the combined activity of the separate components. These antibiotics have until recently been commercially manufactured as feed additives in agriculture, although today there is increased interest in their ability to combat antibiotic-resistant bacteria, particularly vancomycin-resistant bacteria.
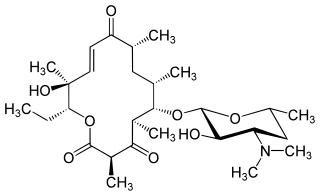
Pikromycin was studied by Brokmann and Hekel in 1951 and was the first antibiotic macrolide to be isolated. Pikromycin is synthesized through a type I polyketide synthase system in Streptomyces venezuelae, a species of Gram-positive bacterium in the genus Streptomyces. Pikromycin is derived from narbonolide, a 14-membered ring macrolide. Along with the narbonolide backbone, pikromycin includes a desosamine sugar and a hydroxyl group. Although Pikromycin is not a clinically useful antibiotic, it can be used as a raw material to synthesize antibiotic ketolide compounds such as ertythromycins and new epothilones.

Codinaeopsin is an antimalarial isolated from a fungal isolate found in white yemeri trees (Vochysia guatemalensis) in Costa Rica. It is reported to have bioactivity against Plasmodium falciparum with an IC50 = 2.3 μg/mL (4.7 μM). Pure codinaeopsin was reported to be isolated with a total yield of 18 mg/mL from cultured fungus. The biosynthesis of codinaeopsin involves a polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS) hybrid.
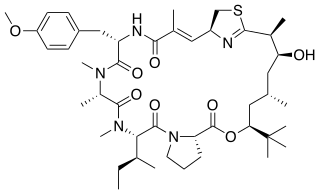
Apratoxin A - is a cyanobacterial secondary metabolite, known as a potent cytotoxic marine natural product. It is a derivative of the Apratoxin family of cytotoxins. The mixed peptide-polyketide natural product comes from a polyketide synthase/non-ribosomal peptide synthase pathway (PKS/NRPS). This cytotoxin is known for inducing G1-phase cell cycle arrest and apoptosis. This natural product's activity has made it a popular target for developing anticancer derivatives.

Pseurotin A is a secondary metabolite of Aspergillus.
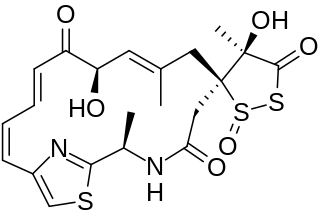
Leinamycin is an 18-membered macrolactam produced by several species of Streptomyces atroolivaceus. This macrolactam has also been shown to exhibit antitumor properties as well as antimicrobial properties against gram-positive and gram-negative bacteria. The presence of a spiro-fused 1,3-dioxo-1,2-dithiolane moiety was a unique structural property at the time of this compound's discovery and it plays an important role in leinamycin's antitumor and antibacterial properties due to its ability to inhibit DNA synthesis.
Curacin A is a hybrid polyketide synthase (PKS)/nonribosomal peptide synthase (NRPS) derived natural product produced isolated from the cyanobacterium Lyngbya majuscula. Curacin A belongs to a family of natural products including jamaicamide, mupirocin, and pederin that have an unusual terminal alkene. Additionally, Curacin A contains a notable thiazoline ring and a unique cyclopropyl moiety, which is essential to the compound's biological activity. Curacin A has been characterized as potent antiproliferative cytotoxic compound with notable anticancer activity for several cancer lines including renal, colon, and breast cancer. Curacin A has been shown to interact with colchicine binding sites on tubulin, which inhibits microtubule polymerization, an essential process for cell division and proliferation.
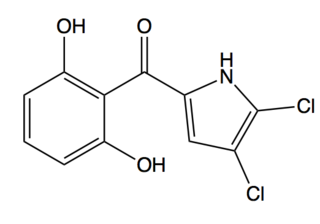
Pyoluteorin is a natural antibiotic that is biosynthesized from a hybrid nonribosomal peptide synthetase (NRPS) and polyketide synthase (PKS) pathway. Pyoluteorin was first isolated in the 1950s from Pseudomonas aeruginosa strains T359 and IFO 3455 and was found to be toxic against oomycetes, bacteria, fungi, and against certain plants. Pyoluteorin is most notable for its toxicity against the oomycete Pythium ultimum, which is a plant pathogen that causes a global loss in agriculture. Currently, pyoluteorin derivatives are being studied as an Mcl-1 antagonist in order to target cancers that have elevated Mcl-1 levels.
Tylactone synthase or TYLS is a Type 1 polyketide synthase. TYLS is found in strains of Streptomyces fradiae and responsible for the synthesis of the macrolide ring, tylactone, the precursor of an antibiotic, tylosin. TYLS is composed of five large multi-functional proteins, TylGI-V. Each protein contains either one or two modules. Each module consists of a minimum of a Ketosynthase (KS), an Acyltransferase (AT), and an Acyl carrier protein (ACP) but may also contain a Ketoreductase (KR), Dehydrotase (DH), and Enoyl Reductase (ER) for additional reduction reactions. The domains of TYLS have similar activity domains to those found in other Type I polyketide synthase such as 6-Deoxyerythronolide B synthase (DEBS). The TYLS system also contains a loading module consisting of a ketosynthase‐like decarboxylase domain, an acyltransferase, and acyl carrier protein. The terminal Thioesterase terminates tylactone synthesis by cyclizing the macrolide ring. After the TYLS completes tylactone synthesis, the tylactone molecule is modified by oxidation at C-20 and C-23 and glycosylation of mycaminose, mycinose, and mycarose to produce tylosin.
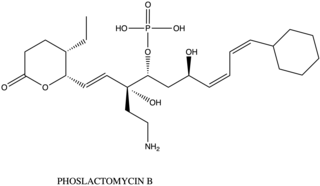
Phoslactomycin (PLM) is a natural product from the isolation of Streptomyces species. This is an inhibitor of the protein serine/threonine phosphatase which is the protein phosphate 2A (PP2A). The PP2A involves the growth factor of the cell such as to induce the formation of mitogen-activated protein interaction and playing a role in cell division and signal transduction. Therefore, PLM is used for the drug that prevents the tumor, cancer, or bacteria. There are nowsaday has 7 kinds of different PLM from PLM A to PLM G which differ the post-synthesis from the biosynthesis of PLM.
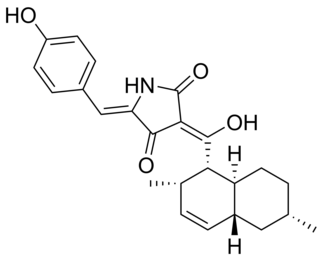
Conipyridoin E is a tetramic acid derivative produced by the fungus Coniochaeta cephalothecoides which was found on a Tibetan Plateau. This natural product has been shown to exhibit antibacterial and antifungal activity against a variety of bacteria, such as Staphylococcus aureus, methicillin-resistant Staphyloccusaureus, and Enterococcus faecalis with MIC50 values of around 0.97 μM. Isolation of a number of analogs of conipyridoin has been accomplished by Han et al. in order to discover novel antibiotic natural products to combat antibiotic resistance.















