1,4-Dichlorobenzene (1,4-DCB, p-DCB, or para-dichlorobenzene, sometimes abbreviated as PDCB or para) is an organic compound with the formula C6H4Cl2. This colorless solid has a strong odor. The molecule consists of a benzene ring with two chlorine atoms (replacing hydrogen atoms) on opposing sites of the ring.

The cumene process is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene, the intermediate material during the process. It was invented by R. Ūdris and P. Sergeyev in 1942 (USSR), and independently by Heinrich Hock in 1944.
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction.

In organic chemistry, a dicarbonyl is a molecule containing two carbonyl groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.
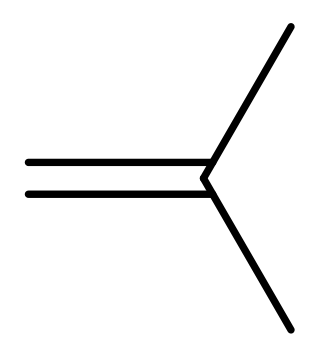
Isobutylene is a hydrocarbon with the chemical formula (CH3)2C=CH2. It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.

Ethylbenzene is an organic compound with the formula C6H5CH2CH3. It is a highly flammable, colorless liquid with an odor similar to that of gasoline. This monocyclic aromatic hydrocarbon is important in the petrochemical industry as a reaction intermediate in the production of styrene, the precursor to polystyrene, a common plastic material. In 2012, more than 99% of ethylbenzene produced was consumed in the production of styrene.
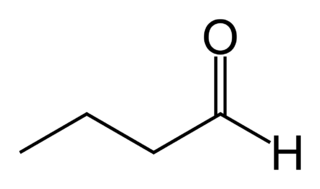
Butyraldehyde, also known as butanal, is an organic compound with the formula CH3(CH2)2CHO. This compound is the aldehyde derivative of butane. It is a colorless flammable liquid with an unpleasant smell. It is miscible with most organic solvents.

Vinylacetylene is the organic compound with the formula C4H4. The colourless gas was once used in the polymer industry. It is composed of both alkyne and alkene groups and is the simplest enyne.

o-Xylene (ortho-xylene) is an aromatic hydrocarbon with the formula C6H4(CH3)2, with two methyl substituents bonded to adjacent carbon atoms of a benzene ring (the ortho configuration). It is a constitutional isomer of m-xylene and p-xylene, the mixture being called xylene or xylenes. o-Xylene is a colourless slightly oily flammable liquid.

Divinylbenzene (DVB) is an organic compound with the chemical formula C6H4(CH=CH2)2 and structure H2C=CH−C6H4−HC=CH2. It is related to styrene by the addition of a second vinyl group. It is a colorless liquid manufactured by the thermal dehydrogenation of isomeric diethylbenzenes. Under synthesis conditions, o-divinylbenzene converts to naphthalene and thus is not a component of the usual mixtures of DVB.
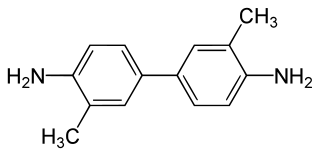
2-Tolidine (orthotolidine, o-tolidine; not to be confused with o-toluidine) is an organic compound with the chemical formula (C6H4(CH3)NH2)2. Several isomers are known; the 3-tolidine derivative is also important commercially. It is a colorless compound although commercial samples are often colored. It is slightly soluble in water. It forms salts with acids, such as the hydrochloride, which is commercially available.
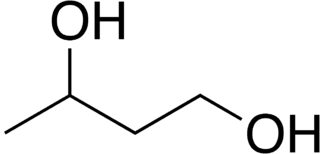
1,3-Butanediol is an organic compound with the formula CH3CH(OH)CH2CH2OH. With two alcohol functional groups, the molecule is classified as a diol. The compound is also chiral, but most studies do not distinguish the enantiomers. The compound is a colorless, bittersweet, water-soluble liquid. It is one of four common structural isomers of butanediol. It is used in flavoring.
In organic chemistry, transalkylation is a chemical reaction involving the transfer of an alkyl group from one organic compound to another. The reaction is used for the transfer of methyl and ethyl groups between benzene rings. This is of particular value in the petrochemical industry to manufacture p-xylene, styrene, and other aromatic compounds. Motivation for using transalkylation reactions is based on a difference in production and demand for benzene, toluene, and xylenes. Transalkylation can convert toluene, which is overproduced, into benzene and xylene, which are under-produced. Zeolites are often used as catalysts in transalkylation reactions.

1-Octanol, also known as octan-1-ol, is the organic compound with the molecular formula CH3(CH2)7OH. It is a fatty alcohol. Many other isomers are also known generically as octanols. 1-Octanol is manufactured for the synthesis of esters for use in perfumes and flavorings. It has a pungent odor. Esters of octanol, such as octyl acetate, occur as components of essential oils. It is used to evaluate the lipophilicity of pharmaceutical products.
Cymene describes organic compounds with the formula CH3C6H4CH(CH3)2. Three isomers exist: 1,2- 1,3-, and 1,4-. All are colorless liquids, immiscible in water, with similar boiling points. They are classified are aromatic hydrocarbons. The bearing two substituents: an isopropyl group and a methyl group.

Diethylbenzene (DEB) refers to any of three isomers with the formula C6H4(C2H5)2. Each consists of a benzene ring and two ethyl substituents. The meta and para have the greater commercial significance. All are colorless liquids.
Ethyltolune describes organic compounds with the formula CH3C6H4CH2CH3. Three isomers exist: 1,2- 1,3-, and 1,4-. All are colorless liquids, immiscible in water, with similar boiling points. They are classified are aromatic hydrocarbons. The ring bears two substituents: a methyl group and an ethyl group.
In industrial chemistry, carboalkoxylation is a process for converting alkenes to esters. This reaction is a form of carbonylation. A closely related reaction is hydrocarboxylation, which employs water in place of alcohols
Bis(2-ethylhexyl) maleate is the chemical compound with the structural formula (H3C 3−CH −CH2−O−C −CH=)2, where the two carboxylate groups are mutually cis. It can be described as the double ester of maleic acid with the alcohol 2-ethylhexanol. It is commonly called dioctyl maleate (DOM), reflecting the older usage of "octane" to refer to any 8-carbon alkane, straight-chained or branched.
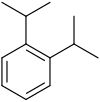
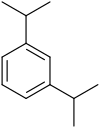
1,3-Diisopropylbenzene is an aromatic hydrocarbon with the formula C6H4(CHMe2)2 (Me = CH3). It is one of three isomeric diisopropylbenzenes. This colorless liquid is prepared by thermal isomerization of 1,4-diisopropylbenzene over a solid acid catalyst. It is the principal industrial precursor to resorcinol via the Hock rearrangement.