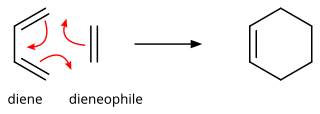
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally-allowed [4+2] cycloaddition with Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reaction becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels-Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.

Benzoin is an organic compound with the formula PhCH(OH)C(O)Ph. It is a hydroxy ketone attached to two phenyl groups. It appears as off-white crystals, with a light camphor-like odor. Benzoin is synthesized from benzaldehyde in the benzoin condensation. It is chiral and it exists as a pair of enantiomers: (R)-benzoin and (S)-benzoin.
The Fritsch–Buttenberg–Wiechell rearrangement, named for Paul Ernst Moritz Fritsch (1859–1913), Wilhelm Paul Buttenberg, and Heinrich G. Wiechell, is a chemical reaction whereby a 1,1-diaryl-2-bromo-alkene rearranges to a 1,2-diaryl-alkyne by reaction with a strong base such as an alkoxide.

The Einhorn–Brunner reaction is the designation for the chemical reaction of imides with alkyl hydrazines to form an isomeric mixture of 1,2,4-triazoles. It was initially described by the German chemist Alfred Einhorn in a paper, published in 1905, describing N-methylol compounds of amides. In 1914 chemist Karl Brunner published a paper expanding on Einhorn's research of the reaction pictured below, thus resulting in the naming as the Einhorn-Brunner. Substituted 1,2,4-triazole have been prepared from diverse imides and hydrazines.

Wilhelm Rudolph Fittig was a German chemist. He discovered the pinacol coupling reaction, mesitylene, diacetyl and biphenyl. Fittig studied the action of sodium on ketones and hydrocarbons. He discovered the Fittig reaction or Wurtz–Fittig reaction for the synthesis of alkylbenzenes, he proposed a diketone structure for benzoquinone and isolated phenanthrene from coal tar. He discovered and synthesized the first lactones and investigated structures of piperine naphthalene and fluorene.

Crotonic acid ((2E)-but-2-enoic acid) is a short-chain unsaturated carboxylic acid, described by the formula CH3CH=CHCO2H. It is called crotonic acid because it was erroneously thought to be a saponification product of croton oil. It crystallizes as colorless needles from hot water. The cis-isomer of crotonic acid is called isocrotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to butyric acid.

The pinacol–pinacolone rearrangement is a method for converting a 1,2-diol to a carbonyl compound in organic chemistry. The 1,2-rearrangement takes place under acidic conditions. The name of the rearrangement reaction comes from the rearrangement of pinacol to pinacolone.
The Lossen rearrangement is the conversion of a hydroxamate ester to an isocyanate. Typically O-acyl, sulfonyl, or phosphoryl O-derivative are employed. The isocyanate can be used further to generate ureas in the presence of amines or generate amines in the presence of H2O.
The Wurtz–Fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Charles Adolphe Wurtz reported what is now known as the Wurtz reaction in 1855, involving the formation of a new carbon-carbon bond by coupling two alkyl halides. Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. This modification of the Wurtz reaction is considered a separate process and is named for both scientists.
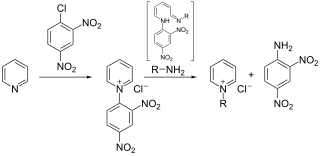
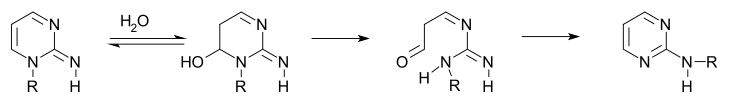
The Zincke reaction is an organic reaction, named after Theodor Zincke, in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine.

Zincke aldehydes, or 5-aminopenta-2,4-dienals, are the product of the reaction of a pyridinium salt with two equivalents of any secondary amine, followed by basic hydrolysis. Using secondary amines the Zincke reaction takes on a different shape forming Zincke aldehydes in which the pyridine ring is ring-opened with the terminal iminium group hydrolyzed to an aldehyde. The use of the dinitrophenyl group for pyridine activation was first reported by Theodor Zincke. The use of cyanogen bromide for pyridine activation was independently reported by W. König:

Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is based on the indole structure, but in which a second benzene ring is fused onto the five-membered ring at the 2–3 position of indole.

The Diels–Reese Reaction is a reaction between hydrazobenzene and dimethyl acetylenedicarboxylate first reported in 1934 by Otto Diels and Johannes Reese. Later work by others extended the reaction scope to include substituted hydrazobenzenes. The exact mechanism is not known. By changing the acidic or basic nature of the solvent, the reaction gives different products. With acetic acid as solvent (acidic), the reaction gives an diphenylpyrazolone. With xylene as solvent (neutral), the reaction gives an indole. With pyridine as solvent (basic), the reaction gives a carbomethoxyquinoline which can be degraded to a dihydroquinoline.

Otto Dimroth was a German chemist. He is known for the Dimroth rearrangement, as well as a type of condenser with an internal double spiral, the Dimroth condenser.

Adolph Strecker was a German chemist who is remembered primarily for his work with amino acids.

The Doebner reaction is the chemical reaction of an aniline with an aldehyde and pyruvic acid to form quinoline-4-carboxylic acids.

The Neber rearrangement is an organic reaction in which a ketoxime is converted into an alpha-aminoketone via a rearrangement reaction.

Phenanthridine is a nitrogen heterocyclic compound that is the basis of DNA-binding fluorescent dyes through intercalation. Examples of such dyes are ethidium bromide and propidium iodide. Acridine is an isomer of phenanthridine.

4-Phenyl-1,2,4-triazoline-3,5-dione (PTAD) is an azodicarbonyl compound. PTAD is one of the strongest dienophiles and reacts rapidly with dienes in Diels-Alder reactions. The most prominent use of PTAD was the first synthesis of prismane in 1973.
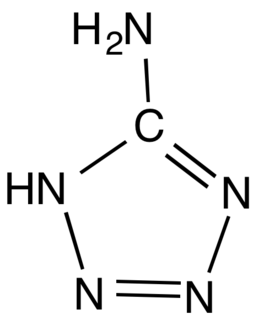
5-Aminotetrazole is an organic compound with the formula HN4CNH2. It is a white solid that can be obtained both in anhydrous and hydrated forms.