
Bioluminescence is the production and emission of light by living organisms. It is a form of chemiluminescence. Bioluminescence occurs widely in marine vertebrates and invertebrates, as well as in some fungi, microorganisms including some bioluminescent bacteria, and terrestrial arthropods such as fireflies. In some animals, the light is bacteriogenic, produced by symbiotic bacteria such as those from the genus Vibrio; in others, it is autogenic, produced by the animals themselves.

Luciferase is a generic term for the class of oxidative enzymes that produce bioluminescence, and is usually distinguished from a photoprotein. The name was first used by Raphaël Dubois who invented the words luciferin and luciferase, for the substrate and enzyme, respectively. Both words are derived from the Latin word lucifer, meaning "lightbearer", which in turn is derived from the Latin words for "light" (lux) and "to bring or carry" (ferre).

Luciferin is a generic term for the light-emitting compound found in organisms that generate bioluminescence. Luciferins typically undergo an enzyme-catalyzed reaction with molecular oxygen. The resulting transformation, which usually involves splitting off a molecular fragment, produces an excited state intermediate that emits light upon decaying to its ground state. The term may refer to molecules that are substrates for both luciferases and photoproteins.

Noctiluca scintillans is a marine species of dinoflagellate that can exist in a green or red form, depending on the pigmentation in its vacuoles. It can be found worldwide, but its geographical distribution varies depending on whether it is green or red. This unicellular microorganism is known for its ability to bioluminesce, giving the water a bright blue glow seen at night. However, blooms of this species can be responsible for environmental hazards, such as toxic red tides. They may also be an indicator of anthropogenic eutrophication.

Aequorin is a calcium-activated photoprotein isolated from the hydrozoan Aequorea victoria. Its bioluminescence was studied decades before the protein was isolated from the animal by Osamu Shimomura in 1962. In the animal, the protein occurs together with the green fluorescent protein to produce green light by resonant energy transfer, while aequorin by itself generates blue light.
Catechol oxidase is a copper oxidase that contains a type 3 di-copper cofactor and catalyzes the oxidation of ortho-diphenols into ortho-quinones coupled with the reduction of molecular oxygen to water. It is present in a variety of species of plants and fungi including Ipomoea batatas and Camellia sinensis. Metalloenzymes with type 3 copper centers are characterized by their ability to reversibly bind dioxygen at ambient conditions. In plants, catechol oxidase plays a key role in enzymatic browning by catalyzing the oxidation of catechol to o-quinone in the presence of oxygen, which can rapidly polymerize to form the melanin that grants damaged fruits their dark brown coloration.

Firefly luciferase is the light-emitting enzyme responsible for the bioluminescence of fireflies and click beetles. The enzyme catalyses the oxidation of firefly luciferin, requiring oxygen and ATP. Because of the requirement of ATP, firefly luciferases have been used extensively in biotechnology.
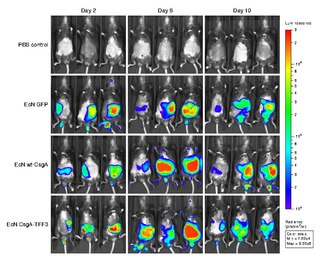
Bioluminescence imaging (BLI) is a technology developed over the past decades (1990's and onward). that allows for the noninvasive study of ongoing biological processes Recently, bioluminescence tomography (BLT) has become possible and several systems have become commercially available. In 2011, PerkinElmer acquired one of the most popular lines of optical imaging systems with bioluminescence from Caliper Life Sciences.
A photocyte is a cell that specializes in catalyzing enzymes to produce light (bioluminescence). Photocytes typically occur in select layers of epithelial tissue, functioning singly or in a group, or as part of a larger apparatus. They contain special structures termed as photocyte granules. These specialized cells are found in a range of multicellular animals including ctenophora, coelenterates (cnidaria), annelids, arthropoda and fishes. Although some fungi are bioluminescent, they do not have such specialized cells.
In enzymology, a Cypridina-luciferin 2-monooxygenase (EC 1.13.12.6) is an enzyme that catalyzes the chemical reaction
In enzymology, an Oplophorus-luciferin 2-monooxygenase, also known as Oplophorus luciferase is a luciferase, an enzyme, from the deep-sea shrimp Oplophorus gracilirostris [2], belonging to a group of coelenterazine luciferases. Unlike other luciferases, it has a broader substrate specificity [3,4,6] and can also bind to bisdeoxycoelenterazine efficiently [3,4]. It is the third example of a luciferase to be purified in lab [2]. The systematic name of this enzyme class is Oplophorus-luciferin:oxygen 2-oxidoreductase (decarboxylating). This enzyme is also called Oplophorus luciferase.
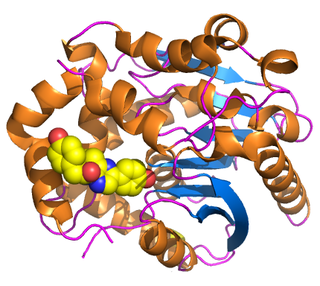
Renilla-luciferin 2-monooxygenase, Renilla luciferase, or RLuc, is a bioluminescent enzyme found in Renilla reniformis, belonging to a group of coelenterazine luciferases. Of this group of enzymes, the luciferase from Renilla reniformis has been the most extensively studied, and due to its bioluminescence requiring only molecular oxygen, has a wide range of applications, with uses as a reporter gene probe in cell culture, in vivo imaging, and various other areas of biological research. Recently, chimeras of RLuc have been developed and demonstrated to be the brightest luminescent proteins to date, and have proved effective in both noninvasive single-cell and whole body imaging.

The enzyme Acid-Induced Arginine Decarboxylase (AdiA), also commonly referred to as arginine decarboxylase, catalyzes the conversion of L-arginine into agmatine and carbon dioxide. The process consumes a proton in the decarboxylation and employs a pyridoxal-5'-phosphate (PLP) cofactor, similar to other enzymes involved in amino acid metabolism, such as ornithine decarboxylase and glutamine decarboxylase. It is found in bacteria and virus, though most research has so far focused on forms of the enzyme in bacteria. During the AdiA catalyzed decarboxylation of arginine, the necessary proton is consumed from the cell cytoplasm which helps to prevent the over-accumulation of protons inside the cell and serves to increase the intracellular pH. Arginine decarboxylase is part of an enzymatic system in Escherichia coli, Salmonella Typhimurium, and methane-producing bacteria Methanococcus jannaschii that makes these organisms acid resistant and allows them to survive under highly acidic medium.

Ubiquitin carboxyl-terminal hydrolase 20 is an enzyme that in humans is encoded by the USP20 gene.
Photoproteins are a type of enzyme, made of protein, from bioluminescent organisms. They add to the function of the luciferins whose usual light-producing reaction is catalyzed by the enzyme luciferase.

John Woodland "Woody" Hastings, was a leader in the field of photobiology, especially bioluminescence, and was one of the founders of the field of circadian biology. He was the Paul C. Mangelsdorf Professor of Natural Sciences and Professor of Molecular and Cellular Biology at Harvard University. He published over 400 papers and co-edited three books.
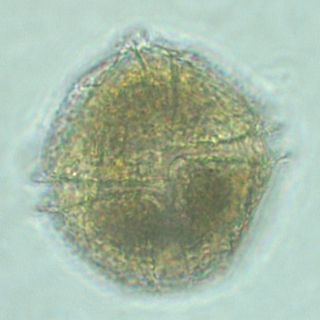
Lingulodinium polyedra is a species of motile photosynthetic dinoflagellates. L. polyedra are often the cause of red tides in southern California, leading to bioluminescent displays on beaches at night.
Atrolysin A is an enzyme that is one of six hemorrhagic toxins found in the venom of western diamondback rattlesnake. This endopeptidase has a length of 419 amino acid residues. The metalloproteinase disintegrin-like domain and the cysteine-rich domain of the enzyme are responsible for the enzyme's hemorrhagic effects on organisms via inhibition of platelet aggregation.

Bioluminescent bacteria are light-producing bacteria that are predominantly present in sea water, marine sediments, the surface of decomposing fish and in the gut of marine animals. While not as common, bacterial bioluminescence is also found in terrestrial and freshwater bacteria. These bacteria may be free living or in symbiosis with animals such as the Hawaiian Bobtail squid or terrestrial nematodes. The host organisms provide these bacteria a safe home and sufficient nutrition. In exchange, the hosts use the light produced by the bacteria for camouflage, prey and/or mate attraction. Bioluminescent bacteria have evolved symbiotic relationships with other organisms in which both participants benefit close to equally. Another possible reason bacteria use luminescence reaction is for quorum sensing, an ability to regulate gene expression in response to bacterial cell density.

Scintillons are small structures in cytoplasm that produce light. Among bioluminescent organisms, only dinoflagellates have scintillons.














