| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Dioxirane | |||
| Systematic IUPAC name Dioxacyclopropane | |||
| Other names 1,2-Dioxacyclopropane Methylene peroxide Peroxymethane | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
PubChem CID | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| CH2O2 | |||
| Molar mass | 46.03 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
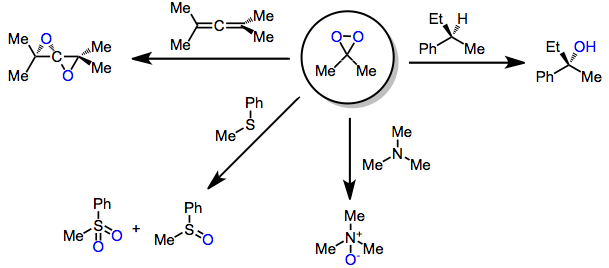
In chemistry, dioxirane (systematically named dioxacyclopropane, also known as methylene peroxide or peroxymethane) is an organic compound with formula CH
2O
2. The molecule consists of a ring with one methylene and two oxygen atoms. It is of interest as the smallest cyclic organic peroxide, but otherwise it is of little practical value.