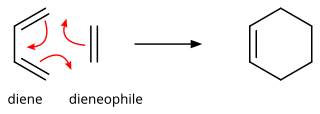
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally allowed [4+2] cycloaddition with Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reaction becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels–Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.

Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet, visible (400–750 nm), or infrared radiation (750–2500 nm).
In organic chemistry, an electrocyclic reaction is a type of pericyclic, rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice versa. These reactions are usually categorized by the following criteria:
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile.
In chemistry, a chemical trap is a chemical compound that is used to detect unstable compounds. The method relies on efficiency of bimolecular reactions with reagents to produce a more easily characterize trapped product. In some cases, the trapping agent is used in large excess.

In chemistry, a phosphaalkyne is an organophosphorus compound containing a triple bond between phosphorus and carbon with the general formula R-C≡P. Phosphaalkynes are the heavier congeners of nitriles, though, due to the similar electronegativities of phosphorus and carbon, possess reactivity patterns reminiscent of alkynes. Due to their high reactivity, phosphaalkynes are not found naturally on earth, but the simplest phosphaalkyne, phosphaethyne (H-C≡P) has been observed in the interstellar medium.
A dendralene is a discrete acyclic cross-conjugated polyene. The simplest dendralene is buta-1,3-diene (1) or [2]dendralene followed by [3]dendralene (2), [4]dendralene (3) and [5]dendralene (4) and so forth. [2]dendralene (butadiene) is the only one not cross-conjugated.
Endohedral hydrogen fullerene (H2@C60) is an endohedral fullerene containing molecular hydrogen. This chemical compound has a potential application in molecular electronics and was synthesized in 2005 at Kyoto University by the group of Koichi Komatsu. Ordinarily the payload of endohedral fullerenes are inserted at the time of the synthesis of the fullerene itself or is introduced to the fullerene at very low yields at high temperatures and high pressure. This particular fullerene was synthesised in an unusual way in three steps starting from pristine C60 fullerene: cracking open the carbon framework, insert hydrogen gas and zipping up by organic synthesis methods.

Hexachlorophosphazene is an inorganic compound with the chemical formula (NPCl2)3. The molecule has a cyclic, unsaturated backbone consisting of alternating phosphorus and nitrogen atoms, and can be viewed as a trimer of the hypothetical compound N≡PCl2. Its classification as a phosphazene highlights its relationship to benzene. There is large academic interest in the compound relating to the phosphorus-nitrogen bonding and phosphorus reactivity.

Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.
The Stieglitz rearrangement is a rearrangement reaction in organic chemistry which is named after the American chemist Julius Stieglitz (1867–1937) and was first investigated by him and Paul Nicholas Leech in 1913. It describes the 1,2-rearrangement of trityl amine derivatives to triaryl imines. It is comparable to a Beckmann rearrangement which also involves a substitution at a nitrogen atom through a carbon to nitrogen shift. As an example, triaryl hydroxylamines can undergo a Stieglitz rearrangement by dehydration and the shift of a phenyl group after activation with phosphorus pentachloride to yield the respective triaryl imine, a Schiff base.
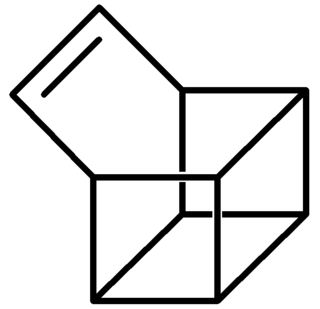
Basketene (IUPAC name: pentacyclo[4.4.0.02,5.03,8.04,7]dec-9-ene) is an organic compound with the formula C10H10. It is a polycyclic alkene and the dehydrogenated version of basketane, which was named for its structural similarity to a basket. Due to its hydrocarbon composition and unique structure, the chemical compound is of considerable interest to those examining energy surfaces of these (CH)10 cage molecules and what possible factors influence their minima. Additionally, the complex structure of this compound has intrigued researchers studying the chemistry of highly strained ring systems. Basketene and its family of derivatives also have important chemical and physical properties. These molecules all tend to have a high standard enthalpy of formation, combined with their high density, leading to possible uses in explosives.
A metal-centered cycloaddition is a subtype of the more general class of cycloaddition reactions. In such reactions "two or more unsaturated molecules unite directly to form a ring", incorporating a metal bonded to one or more of the molecules. Cycloadditions involving metal centers are a staple of organic and organometallic chemistry, and are involved in many industrially-valuable synthetic processes.

Phosphorus mononitride is an inorganic compound with the chemical formula PN. Containing only phosphorus and nitrogen, this material is classified as a binary nitride. From the Lewis structure perspective, it can be represented with a P-N triple bond with a lone pair on each atom. It is isoelectronic with N2, CO, P2, CS and SiO.
Christopher "Kit" Colin Cummins is an American chemist, currently the Henry Dreyfus Professor at the Massachusetts Institute of Technology. He has made contributions to the coordination chemistry of transition metal nitrides, phosphides, and carbides.

1,3-Diphenylisobenzofuran is a highly reactive diene that can scavenge unstable and short-lived dienophiles in a Diels-Alder reaction. It is furthermore used as a standard reagent for the determination of singlet oxygen, even in biological systems. Cycloadditions with 1,3-diphenylisobenzofuran and subsequent oxygen cleavage provide access to a variety of polyaromatics.

Phosphorus monoxide is an unstable radical inorganic compound with molecular formula PO.

Arsenic in the solid state can be found as gray, black, or yellow allotropes. These various forms feature diverse structural motifs, with yellow arsenic enabling the widest range of reactivity. In particular, reaction of yellow arsenic with main group and transition metal elements results in compounds with wide-ranging structural motifs, with butterfly, sandwich and realgar-type moieties featuring most prominently.
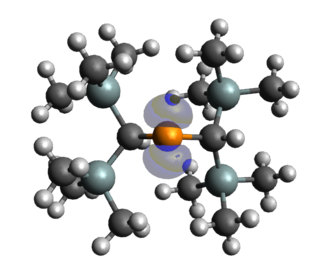
Stable and persistent phosphorus radicals are phosphorus-centred radicals that are isolable and can exist for at least short periods of time. Radicals consisting of main group elements are often very reactive and undergo uncontrollable reactions, notably dimerization and polymerization. The common strategies for stabilising these phosphorus radicals usually include the delocalisation of the unpaired electron over a pi system or nearby electronegative atoms, and kinetic stabilisation with bulky ligands. Stable and persistent phosphorus radicals can be classified into three categories: neutral, cationic, and anionic radicals. Each of these classes involve various sub-classes, with neutral phosphorus radicals being the most extensively studied. Phosphorus exists as one isotope 31P (I = 1/2) with large hyperfine couplings relative to other spin active nuclei, making phosphorus radicals particularly attractive for spin-labelling experiments.
Pnictogen-substituted tetrahedranes are pnictogen-containing analogues of tetrahedranes with the formula RxCxPn4-x. Computational work has indicated that the incorporation of pnictogens to the tetrahedral core alleviates the ring strain of tetrahedrane. Although theoretical work on pnictogen-substituted tetrahedranes has existed for decades, only the phosphorus-containing species have been synthesized. These species exhibit novel reactivities, most often through ring-opening and polymerization pathways. Phosphatetrahedranes are of interest as new retrons for organophosphorus chemistry. Their strain also make them of interest in the development of energy-dense compounds.