
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. The dye is generally applied in an aqueous solution, and may require a mordant to improve the fastness of the dye on the fiber.

Lignin is a class of complex organic polymers that form key structural materials in the support tissues of vascular plants and some algae. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity and do not rot easily. Chemically, lignins are cross-linked phenolic polymers.

Methyl violet is a family of organic compounds that are mainly used as dyes. Depending on the number of attached methyl groups, the color of the dye can be altered. Its main use is as a purple dye for textiles and to give deep violet colors in paint and ink, it is also used as a hydration indicator for silica gel. Methyl violet 10B is also known as crystal violet and has medical uses.

HMG-CoA reductase is the rate-controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. Normally in mammalian cells this enzyme is suppressed by cholesterol derived from the internalization and degradation of low density lipoprotein (LDL) via the LDL receptor as well as oxidized species of cholesterol. Competitive inhibitors of the reductase induce the expression of LDL receptors in the liver, which in turn increases the catabolism of plasma LDL and lowers the plasma concentration of cholesterol, which is considered, by those who accept the standard lipid hypothesis, an important determinant of atherosclerosis. This enzyme is thus the target of the widely available cholesterol-lowering drugs known collectively as the statins.
The Bradford protein assay was developed by Marion M. Bradford in 1976. It is a quick and accurate spectroscopic analytical procedure used to measure the concentration of protein in a solution. The reaction is dependent on the amino acid composition of the measured proteins.

Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl. They are a commercially important family of azo compounds, i.e. compounds containing the linkage C-N=N-C. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related to azo dyes are azo pigments, which are insoluble in water and other solvents.

Orcein, also archil, orchil, lacmus and C.I. Natural Red 28, are names for dyes extracted from several species of lichen, commonly known as "orchella weeds", found in various parts of the world. A major source is the archil lichen, Roccella tinctoria. Orcinol is extracted from such lichens. It is then converted to orcein by ammonia and air. In traditional dye-making methods, urine was used as the ammonia source. If the conversion is carried out in the presence of potassium carbonate, calcium hydroxide, and calcium sulfate, the result is litmus, a more complex molecule. The manufacture was described by Cocq in 1812 and in the UK in 1874. Edmund Roberts noted orchilla as a principal export of the Cape Verde islands, superior to the same kind of "moss" found in Italy or the Canary Islands, that in 1832 was yielding an annual revenue of $200,000. Commercial archil is either a powder or a paste. It is red in acidic pH and blue in alkaline pH.

Solvent Violet 13, also known as D&C Violet No.2, oil violet, Solvent Blue 90, Alizarine Violet 3B, Alizurol Purple, Duranol Brilliant Violet TG, Ahcoquinone Blue IR base, Quinizarin Blue, Disperse Blue 72, and C.I. 60725, is a synthetic anthraquinone dye with bright bluish violet hue. It is a solid insoluble in water and soluble in acetone, toluene, and benzene. Its chemical formula is C21H15NO3, and its structure is 1-hydroxy-4-(p-tolylamino)anthraquinone, or 1-hydroxy-4-[(4-methylphenyl)amino]-9,10-anthracenedione or 1-hydroxy-4-(4-methylanilino)anthraquinone.

β-Hydroxy β-methylglutaryl-CoA (HMG-CoA), also known as 3-hydroxy-3-methylglutaryl-CoA, is an intermediate in the mevalonate and ketogenesis pathways. It is formed from acetyl CoA and acetoacetyl CoA by HMG-CoA synthase. The research of Minor J. Coon and Bimal Kumar Bachhawat in the 1950s at University of Illinois led to its discovery.
Methylcrotonyl CoA carboxylase (MCC) is a biotin-requiring enzyme located in the mitochondria. MCC uses bicarbonate as a carboxyl group source to catalyze the carboxylation of a carbon adjacent to a carbonyl group performing the fourth step in processing leucine, an essential amino acid.
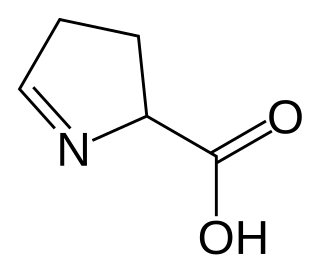
1-Pyrroline-5-carboxylic acid is a cyclic imino acid. Its conjugate base and anion is 1-pyrroline-5-carboxylate (P5C). In solution, P5C is in spontaneous equilibrium with glutamate-5-semialdhyde (GSA). The stereoisomer (S)-1-pyrroline-5-carboxylate is an intermediate metabolite in the biosynthesis and degradation of proline and arginine.

3-Methylglutaconyl-CoA hydratase, also known as MG-CoA hydratase and AUH, is an enzyme encoded by the AUH gene on chromosome 19. It is a member of the enoyl-CoA hydratase/isomerase superfamily, but it is the only member of that family that is able to bind to RNA. Not only does it bind to RNA, AUH has also been observed to be involved in the metabolic enzymatic activity, making it a dual-role protein. Mutations of this gene have been found to cause a disease called 3-Methylglutaconic Acuduria Type 1.
D-amino-acid dehydrogenase is a bacterial enzyme that catalyses the oxidation of D-amino acids into their corresponding oxoacids. It contains both flavin and nonheme iron as cofactors. The enzyme has a very broad specificity and can act on most D-amino acids.

In enzymology, an isovaleryl-CoA dehydrogenase is an enzyme that catalyzes the chemical reaction

In molecular biology, hydroxymethylglutaryl-CoA synthase or HMG-CoA synthase EC 2.3.3.10 is an enzyme which catalyzes the reaction in which acetyl-CoA condenses with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This reaction comprises the second step in the mevalonate-dependent isoprenoid biosynthesis pathway. HMG-CoA is an intermediate in both cholesterol synthesis and ketogenesis. This reaction is overactivated in patients with diabetes mellitus type 1 if left untreated, due to prolonged insulin deficiency and the exhaustion of substrates for gluconeogenesis and the TCA cycle, notably oxaloacetate. This results in shunting of excess acetyl-CoA into the ketone synthesis pathway via HMG-CoA, leading to the development of diabetic ketoacidosis.

α-Ketoisocaproic acid (α-KIC) and its conjugate base, α-ketoisocaproate, are metabolic intermediates in the metabolic pathway for L-leucine. Leucine is an essential amino acid, and its degradation is critical for many biological duties. α-KIC is produced in one of the first steps of the pathway by branched-chain amino acid aminotransferase by transferring the amine on L-leucine onto alpha ketoglutarate, and replacing that amine with a ketone. The degradation of L-leucine in the muscle to this compound allows for the production of the amino acids alanine and glutamate as well. In the liver, α-KIC can be converted to a vast number of compounds depending on the enzymes and cofactors present, including cholesterol, acetyl-CoA, isovaleryl-CoA, and other biological molecules. Isovaleryl-CoA is the main compound synthesized from ɑ-KIC. α-KIC is a key metabolite present in the urine of people with Maple syrup urine disease, along with other branched-chain amino acids. Derivatives of α-KIC have been studied in humans for their ability to improve physical performance during anaerobic exercise as a supplemental bridge between short-term and long-term exercise supplements. These studies show that α-KIC does not achieve this goal without other ergogenicsupplements present as well. α-KIC has also been observed to reduce skeletal muscle damage after eccentrically biased resistance exercises in people who do not usually perform those exercises.
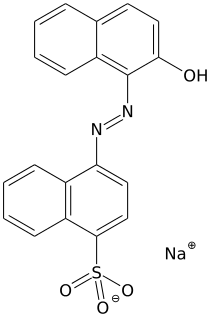
Acid red 88 is an azo dye. Due to its intense colour, solid samples appear almost black. It is used to dye cotton textiles red. A closely related acid dye is Acid Red 13.
Iminocoumarins are a group of chemicals consisting of a coumarin with an attached imine group.
Methine dyes are dyes whose chromophoric system consists of conjugated double bonds (polyenes) flanked by two end groups: an electron acceptor A and an electron donor D.
Structural of methine dyes

Synthetic dyes are found in a wide range of products such as clothes, leather accessories, and furniture. These dyes are commonly used every day. However, a side effect of their widespread use is that up to 12% of these dyes are wasted during the dying process and about 20% of this wastage enters the environment.
















