
Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecules pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid, which crystallizes in needles. There are few commercial applications of acridines; at one time acridine dyes were popular, but they are now relegated to niche applications, such as with acridine orange. The name is a reference to the acrid odour and acrid skin-irritating effect of the compound.

Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.

Azo compounds are organic compounds bearing the functional group diazenyl.

Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C-N=N-C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group, but some dyes contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60-70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents.

Sodium sulfide is a chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both the anhydrous and the hydrated salts in pure crystalline form are colorless solids, although technical grades of sodium sulfide are generally yellow to brick red owing to the presence of polysulfides and commonly supplied as a crystalline mass, in flake form, or as a fused solid. They are water-soluble, giving strongly alkaline solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide, an extremely toxic, flammable and corrosive gas which smells like rotten eggs.

Malononitrile is an organic compound nitrile with the formula CH2(CN)2. It is a colorless or white solid, although aged samples appear yellow or even brown. It is a widely used building block in organic synthesis.
2-Chloroethanol (also called ethylene chlorohydrin or glycol chlorohydrin) is an organic chemical compound with the chemical formula HOCH2CH2Cl and the simplest beta-halohydrin (chlorohydrin). This colorless liquid has a pleasant ether-like odor. It is miscible with water. The molecule is bifunctional, consisting of both an alkyl chloride and an alcohol functional group.

Colored smoke is a kind of smoke created by an aerosol of small particles of a suitable pigment or dye.
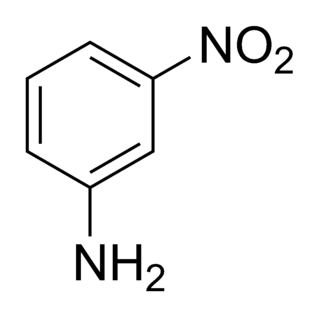
3-Nitroaniline, also known as meta-nitroaniline and m-nitroaniline, is a non-volatile stable solid commonly used as a raw material for dyes. 3-Nitroaniline is an aniline carrying a nitro functional group in position 3. It is stable in neutral, acidic or alkaline solutions and is classified as "not readily biodegradable" with "low bioaccumulation potential".
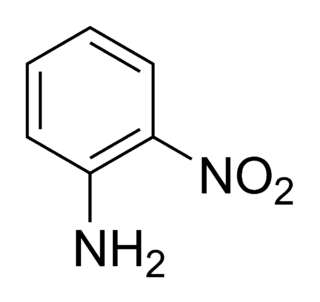
2-Nitroaniline is an organic compound with the formula H2NC6H4NO2. It is a derivative of aniline, carrying a nitro functional group in position 2. It is mainly used as a precursor to o-phenylenediamine.

2,4,6-Trimethylaniline is an organic compound with formula (CH3)3C6H2NH2. It is an aromatic amine that is of commercial interest as a precursor to dyes. It is prepared by selective mononitration of mesitylene, avoiding oxidation of the methyl groups. The resulting nitro compound is reduced to the aniline.

o-Anisidine (2-anisidine) is an organic compound with the formula CH3OC6H4NH2. A colorless liquid, commercial samples can appear yellow owing to air oxidation. It is one of three isomers of the methoxy-containing aniline derivative.
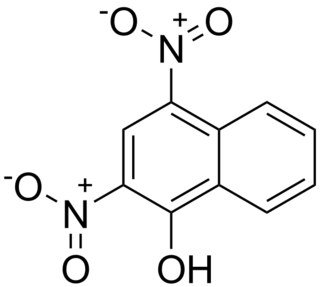
Martius yellow is an organic compound that once was used to protect wool from moths. It is prepared by nitration of naphthol.
1,2-Dichloro-4-nitrobenzene is an organic compound with the formula 1,2-Cl2C6H3-4-NO2. This pale yellow solid is related to 1,2-dichlorobenzene by the replacement of one H atom with a nitro functional group. This compound is an intermediate in the synthesis of agrochemicals.
Disperse dye is a category of synthetic dye intended for polyester and related hydrophobic fibers. Disperse dyes are polar molecules containing anthraquinone or azo groups. It is estimated that 85% of disperse dyes are azos or anthraquinone dyes.
4-Nitrotoluene or para-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is a pale yellow solid. It is one of three isomers of nitrotoluene.
3-Nitrotoluene or meta-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is one of three isomers of nitrotoluene. A yellow liquid, it is used in the manufacture of meta-toluidine, which is an intermediate in the production of various dyes.
2-Nitrotoluene or ortho-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is pale yellow liquid that crystallizes in two forms, called α (−9.27 °C) and β (−3.17 °C). It is mainly a precursor to o-toluidine, which is an intermediate in the production of various dyes.
2,4-Dinitroaniline is a chemical compound with a formula of C6H5N3O4. It is used as an explosive and as a reagent to detect and characterize aldehydes and ketones.
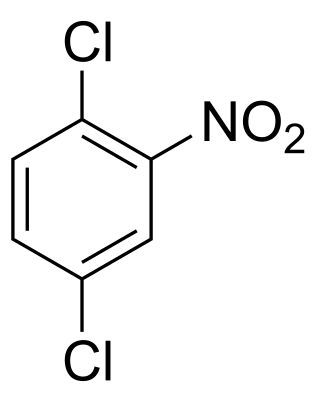
1,4-Dichloro-2-nitrobenzene is an organic compound with the formula C6H3Cl2NO2. One of several isomers of dichloronitrobenzene, it is a yellow solid that is insoluble in water. It is produced by nitration of 1,4-dichlorobenzene. It is a precursor to many derivatives of commercial interest. Hydrogenation gives 1,4-dichloroaniline. Nucleophiles displace the chloride adjacent to the nitro group: ammonia gives the aniline derivative, aqueous base gives the phenol derivative, and methoxide gives the anisole derivative. These compounds are respectively 4-chloro-2-nitroaniline, 4-chloro-2-nitrophenol, and 4-chloro-2-nitroanisole.















