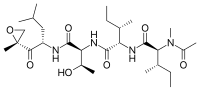
Native chemical ligation is an important extension of the chemical ligation concept for constructing a larger polypeptide chain by the covalent condensation of two or more unprotected peptides segments. Native chemical ligation is the most effective method for synthesizing native or modified proteins of typical size.
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: total synthesis, semisynthesis, and methodology.
Chemical biology is a scientific discipline spanning the fields of chemistry and biology. The discipline involves the application of chemical techniques, analysis, and often small molecules produced through synthetic chemistry, to the study and manipulation of biological systems. In contrast to biochemistry, which involves the study of the chemistry of biomolecules and regulation of biochemical pathways within and between cells, chemical biology deals with chemistry applied to biology.
In chemical synthesis, click chemistry is a class of biocompatible small molecule reactions commonly used in bioconjugation, allowing the joining of substrates of choice with specific biomolecules. Click chemistry is not a single specific reaction, but describes a way of generating products that follow examples in nature, which also generates substances by joining small modular units. In many applications, click reactions join a biomolecule and a reporter molecule. Click chemistry is not limited to biological conditions: the concept of a "click" reaction has been used in chemoproteomic, pharmacological, and various biomimetic applications. However, they have been made notably useful in the detection, localization and qualification of biomolecules.

Alkyne metathesis is an organic reaction that entails the redistribution of alkyne chemical bonds. The reaction requires metal catalysts. Mechanistic studies show that the conversion proceeds via the intermediacy of metal alkylidyne complexes. The reaction is related to olefin metathesis.
Ring-closing metathesis (RCM) is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.
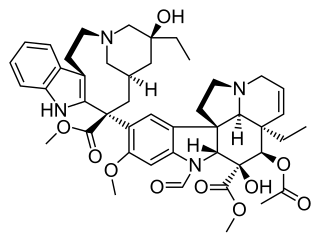
Vinca alkaloids are a set of anti-mitotic and anti-microtubule alkaloid agents originally derived from the periwinkle plant Catharanthus roseus and other vinca plants. They block beta-tubulin polymerization in a dividing cell.

Danishefsky's diene is an organosilicon compound and a diene with the formal name trans-1-methoxy-3-trimethylsilyloxy-buta-1,3-diene named after Samuel J. Danishefsky. Because the diene is very electron-rich it is a very reactive reagent in Diels-Alder reactions. This diene reacts rapidly with electrophilic alkenes, such as maleic anhydride. The methoxy group promotes highly regioselective additions. The diene is known to react with amines, aldehydes, alkenes and alkynes. Reactions with imines and nitro-olefins have been reported.
Isoindoline is a heterocyclic organic compound with the molecular formula C8H9N. The parent compound has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound's structure is similar to indoline except that the nitrogen atom is in the 2 position instead of the 1 position of the five-membered ring. Isoindoline itself is not commonly encountered, but several derivatives are found in nature and some synthetic derivatives are commercially valuable drugs, e.g. pazinaclone.

Migrastatin is an organic compound which naturally occurs in the Streptomyces platensis bacteria. Migrastatin and several of its analogues have shown to have potential in treating cancer, as it inhibits the metastasis of cancer cells.

Indolocarbazoles (ICZs) are a class of compounds that are under current study due to their potential as anti-cancer drugs and the prospective number of derivatives and uses found from the basic backbone alone. First isolated in 1977, a wide range of structures and derivatives have been found or developed throughout the world. Due to the extensive number of structures available, this review will focus on the more important groups here while covering their occurrence, biological activity, biosynthesis, and laboratory synthesis.

Indole is an aromatic heterocyclic organic compound with the formula C8H7N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin.

Pleuromutilin and its derivatives are antibacterial drugs that inhibit protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes.
The Saegusa–Ito oxidation is a chemical reaction used in organic chemistry. It was discovered in 1978 by Takeo Saegusa and Yoshihiko Ito as a method to introduce α-β unsaturation in carbonyl compounds. The reaction as originally reported involved formation of a silyl enol ether followed by treatment with palladium(II) acetate and benzoquinone to yield the corresponding enone. The original publication noted its utility for regeneration of unsaturation following 1,4-addition with nucleophiles such as organocuprates.
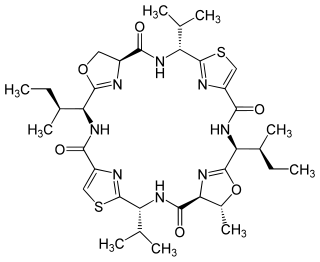
Patellamide A is a peptide natural product produced by Prochloron didemni, a cyanobacterial symbiont of Lissoclinum patella, and was first isolated in 1981. Patellamide A is one of many didemnid peptides. Other closely related peptides include patellamides B, C, and D and trunkamide. The patellamides and trunkamide show moderate cytotoxicity and activity against multidrug resistant cancer cell lines.
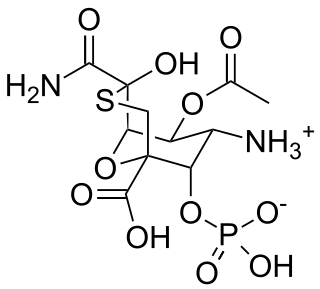
Tagetitoxin (TGT) is a bacterial phytotoxin produced by Pseudomonas syringae pv. tagetis.
Biomimetic synthesis is an area of organic chemical synthesis that is specifically biologically inspired. The term encompasses both the testing of a "biogenetic hypothesis" through execution of a series of reactions designed to parallel the proposed biosynthesis, as well as programs of study where a synthetic reaction or reactions aimed at a desired synthetic goal are designed to mimic one or more known enzymic transformations of an established biosynthetic pathway. The earliest generally cited example of a biomimetic synthesis is Sir Robert Robinson's organic synthesis of the alkaloid tropinone.
Lactimidomycin is a glutarimide antibiotic derived from the bacteria Streptomyces amphibiosporus. It has antifungal, antiviral and anti-cancer properties, acting as a direct inhibitor of protein translation in ribosomes. Antiviral activity is seen against a variety of RNA viruses including flaviviruses such as dengue fever, Kunjin virus and Modoc virus, as well as vesicular stomatitis virus and poliovirus. As lactimidomycin is a natural product containing an unusual unsaturated 12-membered lactone ring, it has been the subject of numerous total synthesis approaches.

James Inglese is an American biochemist, the director of the Assay Development and Screening Technology laboratory at the National Center for Advancing Translational Sciences, a Center within the National Institutes of Health. His specialty is small molecule high throughput screening. Inglese's laboratory develops methods and strategies in molecular pharmacology with drug discovery applications. The work of his research group and collaborators focuses on genetic and infectious disease-associated biology.

Eric Block is an American chemist whose research has focused on the chemistry of organosulfur and organoselenium compounds, Allium chemistry, and the chemistry of olfaction. As of 2018, he is Distinguished Professor of Chemistry Emeritus at the University at Albany, SUNY.