
Catalysis is the increase in rate of a chemical reaction due to an added substance known as a catalyst. Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst.
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the general subject of organic synthesis, there are many different types of synthetic routes that can be completed including total synthesis, stereoselective synthesis, automated synthesis, and many more. Additionally, in understanding organic synthesis it is necessary to be familiar with the methodology, techniques, and applications of the subject.

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts."
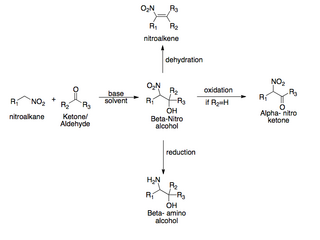
The Henry reaction is a classic carbon–carbon bond formation reaction in organic chemistry. Discovered in 1895 by the Belgian chemist Louis Henry (1834–1913), it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-nitro alcohols. This type of reaction is also referred to as a nitroaldol reaction. It is nearly analogous to the aldol reaction that had been discovered 23 years prior that couples two carbonyl compounds to form β-hydroxy carbonyl compounds known as "aldols". The Henry reaction is a useful technique in the area of organic chemistry due to the synthetic utility of its corresponding products, as they can be easily converted to other useful synthetic intermediates. These conversions include subsequent dehydration to yield nitroalkenes, oxidation of the secondary alcohol to yield α-nitro ketones, or reduction of the nitro group to yield β-amino alcohols.
Homochirality is a uniformity of chirality, or handedness. Objects are chiral when they cannot be superposed on their mirror images. For example, the left and right hands of a human are approximately mirror images of each other but are not their own mirror images, so they are chiral. In biology, 19 of the 20 natural amino acids are homochiral, being L-chiral (left-handed), while sugars are D-chiral (right-handed). Homochirality can also refer to enantiopure substances in which all the constituents are the same enantiomer, but some sources discourage this use of the term.

Biocatalysis refers to the use of living (biological) systems or their parts to speed up (catalyze) chemical reactions. In biocatalytic processes, natural catalysts, such as enzymes, perform chemical transformations on organic compounds. Both enzymes that have been more or less isolated and enzymes still residing inside living cells are employed for this task. Modern biotechnology, specifically directed evolution, has made the production of modified or non-natural enzymes possible. This has enabled the development of enzymes that can catalyze novel small molecule transformations that may be difficult or impossible using classical synthetic organic chemistry. Utilizing natural or modified enzymes to perform organic synthesis is termed chemoenzymatic synthesis; the reactions performed by the enzyme are classified as chemoenzymatic reactions.

In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a misnomer for enzymes due to their comparable effects on reaction rates and forms of catalysis involved.
The Hajos–Parrish–Eder–Sauer–Wiechert and Barbas-List reactions in organic chemistry are a family of proline-catalysed asymmetric aldol reactions.
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen to a target (substrate) molecule with three-dimensional spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate many novel synthetic molecules that interact with biological systems in specific ways, leading to new pharmaceutical agents and agrochemicals. The importance of asymmetric hydrogenation in both academia and industry contributed to two of its pioneers — William Standish Knowles and Ryōji Noyori — being collectively awarded one half of the 2001 Nobel Prize in Chemistry.
Masakatsu Shibasaki is a Japanese chemist. In 1974 he earned his doctorate in chemistry, in the group of Shun’ichi Yamada. He did a post doc with Elias J. Corey at Harvard. He returned to Japan and became a professor in 1977 at Teikyō University and moved to Hokkaidō University 1986. 1983–1986 Shibasaki was a research group leader at the Sagami chemical research center. From 1991 until 2010 he served as professor at Tokyo University. Since 2010 he is representative director of Microbial Chemistry Research Foundation (Chemistry), Tokyo. He is perhaps best known for developing a range of binol based heterobimetallic catalysts, which now bear his name.

In organic chemistry, the Soai reaction is the alkylation of pyrimidine-5-carbaldehyde with diisopropylzinc. The reaction is autocatalytic and leads to rapidly increasing amounts of the same enantiomer of the product. The product pyrimidyl alcohol is chiral and induces that same chirality in further catalytic cycles. Starting with a low enantiomeric excess ("ee") produces a product with very high enantiomeric excess. The reaction has been studied for clues about the origin of homochirality among certain classes of biomolecules.
Spontaneous absolute asymmetric synthesis is a chemical phenomenon that stochastically generates chirality based on autocatalysis and small fluctuations in the ratio of enantiomers present in a racemic mixture. In certain reactions which initially do not contain chiral information, stochastically distributed enantiomeric excess can be observed. The phenomenon is different from chiral amplification, where enantiomeric excess is present from the beginning and not stochastically distributed. Hence, when the experiment is repeated many times, the average enantiomeric excess approaches 0%. The phenomenon has important implications concerning the origin of homochirality in nature.
In enantioselective synthesis, a non-linear effect refers to a process in which the enantiopurity of the catalyst or chiral auxiliary does not correspond with the enantiopurity of the product produced. For example: a racemic catalyst would be expected to convert a prochiral substrate into a racemic product, but this is not always the case and a chirally enriched product can be produced instead.
In chemistry, reaction progress kinetic analysis (RPKA) is a subset of a broad range of kinetic techniques utilized to determine the rate laws of chemical reactions and to aid in elucidation of reaction mechanisms. While the concepts guiding reaction progress kinetic analysis are not new, the process was formalized by Professor Donna Blackmond in the late 1990s and has since seen increasingly widespread use. Unlike more common pseudo-first-order analysis, in which an overwhelming excess of one or more reagents is used relative to a species of interest, RPKA probes reactions at synthetically relevant conditions Generally, this analysis involves a system in which the concentrations of multiple reactants are changing measurably over the course of the reaction. As the mechanism can vary depending on the relative and absolute concentrations of the species involved, this approach obtains results that are much more representative of reaction behavior under commonly utilized conditions than do traditional tactics. Furthermore, information obtained by observation of the reaction over time may provide insight regarding unexpected behavior such as induction periods, catalyst deactivation, or changes in mechanism.
Proline organocatalysis is the use of proline as an organocatalyst in organic chemistry. This theme is often considered the starting point for the area of organocatalysis, even though early discoveries went unappreciated. Modifications, such as MacMillan’s catalyst and Jorgensen's catalysts, proceed with excellent stereocontrol.
Clark Landis is an American chemist, whose research focuses on organic and inorganic chemistry. He is currently a Professor of Chemistry at the University of Wisconsin–Madison. He was awarded the ACS Award in Organometallic Chemistry in 2010, and is a fellow of the American Chemical Society and the American Association for the Advancement of Science.
In homogeneous catalysis, C2-symmetric ligands refer to ligands that lack mirror symmetry but have C2 symmetry. Such ligands are usually bidentate and are valuable in catalysis. The C2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. C2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including C2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product.
Konstantin P. Bryliakov is a Russian chemist and author of monographs and over 170 research papers, textbooks, and patents. He is a professor at Russian Academy of Sciences and Novosibirsk State University.
Thomas Lectka is an American organic chemist, academic and researcher. He is Jean and Norman Scowe Professor of Chemistry and leads the Lectka Group at Johns Hopkins University.

Jordi Burés is a Full Professor of Organic Chemistry in the Department of Chemistry at The University of Manchester. His research in general is on the areas of organic and physical chemistry, specializing in Mechanistic Studies, nuclear magnetic resonance and catalysis.






