
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are a class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from virucides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural virucides are produced by some plants such as eucalyptus and Australian tea trees.

Dicer, also known as endoribonuclease Dicer or helicase with RNase motif, is an enzyme that in humans is encoded by the DICER1 gene. Being part of the RNase III family, Dicer cleaves double-stranded RNA (dsRNA) and pre-microRNA (pre-miRNA) into short double-stranded RNA fragments called small interfering RNA and microRNA, respectively. These fragments are approximately 20–25 base pairs long with a two-base overhang on the 3′-end. Dicer facilitates the activation of the RNA-induced silencing complex (RISC), which is essential for RNA interference. RISC has a catalytic component Argonaute, which is an endonuclease capable of degrading messenger RNA (mRNA).

Wolbachia is a genus of gram-negative bacteria that can either infect many species of arthropod as an intracellular parasite, or act as a mutualistic microbe in filarial nematodes. It is one of the most common parasitic microbes of arthropods, and is possibly the most common reproductive parasite in the biosphere. Its interactions with its hosts are often complex. Some host species cannot reproduce, or even survive, without Wolbachia colonisation. One study concluded that more than 16% of neotropical insect species carry bacteria of this genus, and as many as 25 to 70% of all insect species are estimated to be potential hosts.

Polydnaviriformidae ( PDV) is a family of insect viriforms; members are known as polydnaviruses. There are two genera in the family: bracoform and Ichnoviriform. Polydnaviruses form a symbiotic relationship with parasitoid wasps. Ichnoviriforms (IV) occur in Ichneumonid wasps and Bracoviriforms (BV) in Braconid wasps. The larvae of wasps in both of those groups are themselves parasitic on Lepidoptera, and the polydnaviruses are important in circumventing the immune response of their parasitized hosts. Little or no sequence homology exists between BV and IV, suggesting that the two genera have been evolving independently for a long time.

Drosophila simulans is a species of fly closely related to D. melanogaster, belonging to the same melanogaster species subgroup. Its closest relatives are D. mauritiana and D. sechellia.
Balancer chromosomes are a type of genetically engineered chromosome used in laboratory biology for the maintenance of recessive lethal mutations within living organisms without interference from natural selection. Since such mutations are viable only in heterozygotes, they cannot be stably maintained through successive generations and therefore continually lead to production of wild-type organisms, which can be prevented by replacing the homologous wild-type chromosome with a balancer. In this capacity, balancers are crucial for genetics research on model organisms such as Drosophila melanogaster, the common fruit fly, for which stocks cannot be archived. They can also be used in forward genetics screens to specifically identify recessive lethal mutations. For that reason, balancers are also used in other model organisms, most notably the nematode worm Caenorhabditis elegans and the mouse.
Piwi-interacting RNA (piRNA) is the largest class of small non-coding RNA molecules expressed in animal cells. piRNAs form RNA-protein complexes through interactions with piwi-subfamily Argonaute proteins. These piRNA complexes are mostly involved in the epigenetic and post-transcriptional silencing of transposable elements and other spurious or repeat-derived transcripts, but can also be involved in the regulation of other genetic elements in germ line cells.
Drosophila X virus (DXV) belongs to the Birnaviridae family of viruses. Birnaviridae currently consists of three genera. The first genus is Entomobirnavirus, which contains DXV. The next genus is Aquabirnavirus, containing infectious pancreatic necrosis virus (IPNV). The last genus is Avibirnavirus, which contains infectious bursal disease virus (IBDV). All of these genera contain homology in three specific areas of their transcripts. The homology comes from the amino and carboxyl regions of preVP2, a small 21-residue-long domain near the carboxyl terminal of VP3, and similar small ORFs sequences.

Most animal testing involves invertebrates, especially Drosophila melanogaster, a fruit fly, and Caenorhabditis elegans, a nematode. These animals offer scientists many advantages over vertebrates, including their short life cycle, simple anatomy and the ease with which large numbers of individuals may be studied. Invertebrates are often cost-effective, as thousands of flies or nematodes can be housed in a single room.

Mosquito-borne diseases or mosquito-borne illnesses are diseases caused by bacteria, viruses or parasites transmitted by mosquitoes. Nearly 700 million people get a mosquito-borne illness each year, resulting in over 725,000 deaths.

Nudiviruses are a family of animal viruses that constitute the family Nudiviridae. Insects and marine crustaceans serve as natural hosts. There are 11 species in this family, assigned to 4 genera. Diseases associated with this family include: death in larvae, chronic disease in adults.
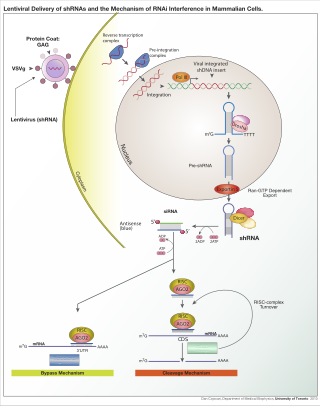
RNA interference (RNAi) is a biological process in which RNA molecules are involved in sequence-specific suppression of gene expression by double-stranded RNA, through translational or transcriptional repression. Historically, RNAi was known by other names, including co-suppression, post-transcriptional gene silencing (PTGS), and quelling. The detailed study of each of these seemingly different processes elucidated that the identity of these phenomena were all actually RNAi. Andrew Fire and Craig C. Mello shared the 2006 Nobel Prize in Physiology or Medicine for their work on RNAi in the nematode worm Caenorhabditis elegans, which they published in 1998. Since the discovery of RNAi and its regulatory potentials, it has become evident that RNAi has immense potential in suppression of desired genes. RNAi is now known as precise, efficient, stable and better than antisense therapy for gene suppression. Antisense RNA produced intracellularly by an expression vector may be developed and find utility as novel therapeutic agents.
Hytrosaviridae is a family of double-stranded DNA viruses that infect insects. The name is derived from Hytrosa, sigla from the Greek Hypertrophia for 'hypertrophy' and 'sialoadenitis' for 'salivary gland inflammation.'
sCAR-Fc is an experimental prophylactic treatment against coxsackievirus B3 (CVB) infections. Coxsackievirus B3 can cause cardiac damage, eventually resulting in a weakened and enlarged heart that is termed dilated cardiomyopathy. While many other treatments inhibit viral proliferation in myocytes, sCAR-Fc prevents the virus entering the cell by competitively binding to coxsackie virus and adenovirus receptors (CAR) on the membrane of myocytes.
An interferon-stimulated gene (ISG) is a gene that can be expressed in response to stimulation by interferon. Interferons bind to receptors on the surface of a cell, initiating protein signaling pathways within the cell. This interaction leads to the expression of a subset of genes involved in the innate immune system response. ISGs are commonly expressed in response to viral infection, but also during bacterial infection and in the presence of parasites. It's currently estimated that 10% of the human genome is regulated by interferons (IFNs). Interferon stimulated genes can act as an initial response to pathogen invasion, slowing down viral replication and increasing expression of immune signaling complexes. There are three known types of interferon. With approximately 450 genes highly expressed in response to interferon type I. Type I interferon consists of INF-α, INF-β, INF-ω and is expressed in response to viral infection. ISGs induced by type I interferon are associated with viral replication suppression and increase expression of immune signaling proteins. Type II interferon consists only of INF-γ and is associated with controlling intracellular pathogens and tumor suppressor genes. Type III interferon consists of INF-λ and is associated with viral immune response and is key in anti-fungal neutrophil response.

Caenorhabditis elegans- microbe interactions are defined as any interaction that encompasses the association with microbes that temporarily or permanently live in or on the nematode C. elegans. The microbes can engage in a commensal, mutualistic or pathogenic interaction with the host. These include bacterial, viral, unicellular eukaryotic, and fungal interactions. In nature C. elegans harbours a diverse set of microbes. In contrast, C. elegans strains that are cultivated in laboratories for research purposes have lost the natural associated microbial communities and are commonly maintained on a single bacterial strain, Escherichia coli OP50. However, E. coli OP50 does not allow for reverse genetic screens because RNAi libraries have only been generated in strain HT115. This limits the ability to study bacterial effects on host phenotypes. The host microbe interactions of C. elegans are closely studied because of their orthologs in humans. Therefore, the better we understand the host interactions of C. elegans the better we can understand the host interactions within the human body.
Sigmavirus is a genus of viruses in the family Rhabdoviridae, order Mononegavirales. Sigmaviruses naturally infect dipterans. It is not to be confused with the Mega Man character of the same name.
Flock House virus (FHV) is in the Alphanodavirus genus of the Nodaviridae family of viruses. Flock House virus was isolated from a grass grub at the Flock House research station in Bulls, New Zealand. FHV is an extensively studied virus and is considered a model system for the study of other non-enveloped RNA viruses owing to its small size and genetic tractability, particularly to study the role of the transiently exposed hydrophobic gamma peptide and the metastability of the viral capsid. FHV can be engineered in insect cell culture allowing for the tailored production of native or mutant authentic virions or virus-like-particles. FHV is a platform for nanotechnology and nanomedicine, for example, for epitope display and vaccine development. Viral entry into host cells occurs via receptor-mediated endocytosis. Receptor binding initiates a sequence of events during which the virus exploits the host environment in order to deliver the viral cargo in to the host cytosol. Receptor binding prompts the meta-stability of the capsid–proteins, the coordinated rearrangements of which are crucial for subsequent steps in the infection pathway. In addition, the transient exposure of a covalently-independent hydrophobic γ-peptide is responsible for breaching cellular membranes and is thus essential for the viral entry of FHV into host cells.

The Drosophila quinaria species group is a speciose lineage of mushroom-feeding flies studied for their specialist ecology, their parasites, population genetics, and the evolution of immune systems. Quinaria species are part of the Drosophila subgenus.

Drosophila innubila is a species of vinegar fly restricted to high-elevation woodlands in the mountains of the southern USA and Mexico, which it likely colonized during the last glacial period. Drosophila innubila is a kind of mushroom-breeding Drosophila, and member of the Drosophila quinaria species group. Drosophila innubila is best known for its association with a strain of male-killing Wolbachia bacteria. These bacteria are parasitic, as they drain resources from the host and cause half the infected female's eggs to abort. However Wolbachia may offer benefits to the fly's fitness in certain circumstances. The D. innubila genome was sequenced in 2019.










