
Tacrolimus, sold under the brand names Protopic and Prograf among others, is an immunosuppressive drug. After allogeneic organ transplant, the risk of organ rejection is moderate. To lower the risk of organ rejection, Tacrolimus is given. The drug can also be sold as a topical medication in the treatment of T-cell-mediated diseases such as eczema and psoriasis. For example, it is prescribed for severe refractory uveitis after a bone marrow transplant, exacerbations of minimal change disease, Kimura's disease, and vitiligo. It can be used to treat dry eye syndrome in cats and dogs.
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.

Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same.
Biological half-life of a biological substance such as medication is the time it takes from its maximum concentration (Cmax) to half maximum concentration in human body, and is denoted by the abbreviation .
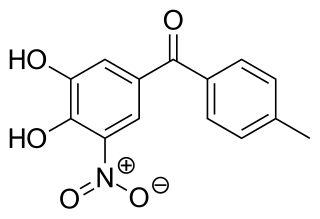
Tolcapone, sold under the brand name Tasmar, is a medication used to treat Parkinson's disease (PD). It is a selective, potent and reversible nitrocatechol-type inhibitor of the enzyme catechol-O-methyltransferase (COMT). It has demonstrated significant liver toxicity, which has led to suspension of marketing authorisations in a number of countries.

Acecainide is an antiarrhythmic drug. Chemically, it is the N-acetylated metabolite of procainamide. It is a Class III antiarrhythmic agent, whereas procainamide is a Class Ia antiarrhythmic drug. It is only partially as active as procainamide; when checking levels, both must be included in the final calculation.
Pharmacokinetics, sometimes abbreviated as PK, is a branch of pharmacology dedicated to determine the fate of substances administered to a living organism. The substances of interest include any chemical xenobiotic such as: pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is the study of how an organism affects a drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models.

Pazopanib, sold under the brand name Votrient, is an anti-cancer medication. It is a potent and selective multi-targeted receptor tyrosine kinase inhibitor that blocks tumour growth and inhibits angiogenesis. It has been approved for renal cell carcinoma and soft tissue sarcoma by numerous regulatory administrations worldwide.

Vernakalant is a pharmaceutical drug for the acute conversion of atrial fibrillation, a kind of irregular heartbeat, in form of an intravenous infusion. It has been approved for use in the European Union and the UK since 2010. The US Food and Drug Administration denied approval in 2008 and 2019.

Teriflunomide, sold under the brand name Aubagio, is the active metabolite of leflunomide. Teriflunomide was investigated in the Phase III clinical trial TEMSO as a medication for multiple sclerosis (MS). The study was completed in July 2010. 2-year results were positive. However, the subsequent TENERE head-to-head comparison trial reported that "although permanent discontinuations [of therapy] were substantially less common among MS patients who received teriflunomide compared with interferon beta-1a, relapses were more common with teriflunomide." The drug was approved for use in the United States in September 2012 and for use in the European Union in August 2013.
Cmin is a term used in pharmacokinetics for the minimum blood plasma concentration reached by a drug prior to administration of a second dose. Cmin is the opposite of Cmax, the maximum concentration that the drug reaches. Cmin must be above certain thresholds, such as the minimum inhibitory concentration (MIC), to achieve a therapeutic effect.
Cmax is the maximum serum concentration that a drug achieves in a specified compartment or test area of the body after the drug has been administered and before the administration of a second dose. It is a standard measurement in pharmacokinetics.
In the field of pharmacokinetics, the area under the curve (AUC) is the definite integral of a curve that describes the variation of a drug concentration in blood plasma as a function of time. In practice, the drug concentration is measured at certain discrete points in time and the trapezoidal rule is used to estimate AUC.
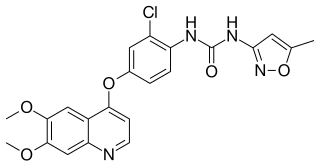
Tivozanib, sold under the brand name Fotivda, is a medication used for the treatment of relapsed or refractory advanced renal cell carcinoma (RCC). It is an oral VEGF receptor tyrosine kinase inhibitor. It was discovered by Kyowa Kirin and developed by AVEO Pharmaceuticals.

Dapoxetine, marketed as Priligy, among others, is a medication used for the treatment of premature ejaculation (PE) in men 18–64 years old. Dapoxetine works by inhibiting the serotonin transporter, increasing serotonin's action at the postsynaptic cleft, and as a consequence promoting ejaculatory delay. As a member of the selective serotonin reuptake inhibitor (SSRI) family, dapoxetine was initially created as an antidepressant. However, unlike other SSRIs, dapoxetine is absorbed and eliminated rapidly in the body. Its fast-acting property makes it suitable for the treatment of PE, but not as an antidepressant.
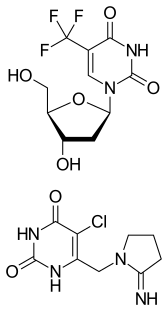
Trifluridine/tipiracil, sold under the brand name Lonsurf, is a fixed-dose combination medication that is used as a third- or fourth-line treatment of metastatic colorectal cancer or gastric cancer, after chemotherapy and targeted therapeutics have failed. It is a combination of two active pharmaceutical ingredients: trifluridine, a nucleoside analog, and tipiracil, a thymidine phosphorylase inhibitor. Tipiracil prevents rapid metabolism of trifluridine, increasing the bioavailability of trifluridine.

Eluxadoline, sold under the brand names Viberzi and Truberzi, is a medication taken by mouth for the treatment of diarrhea and abdominal pain in individuals with diarrhea-predominant irritable bowel syndrome (IBS-D). It was approved for use in the United States in 2015. The drug originated from Janssen Pharmaceutica and was developed by Actavis.
The residence time of a fluid parcel is the total time that the parcel has spent inside a control volume. The residence time of a set of parcels is quantified in terms of the frequency distribution of the residence time in the set, which is known as residence time distribution (RTD), or in terms of its average, known as mean residence time.

Upadacitinib, sold under the brand name Rinvoq, is a Janus kinase (JAK) inhibitor medication for the treatment of moderately to severely active rheumatoid arthritis in adults where methotrexate did not work well or could not be tolerated. It was approved for medical use in the United States and in the European Union in 2019, and was developed by the biotech company AbbVie.

Ertugliflozin is a drug for the treatment of type 2 diabetes. In the United States, it was approved by the Food and Drug Administration for use as a monotherapy and as a fixed dose combination with either sitagliptin or with metformin. In Europe, it was approved in March 2018, for use as a monotherapy or combination therapy. In September 2020, The New England Journal of Medicine reported that ertugliflozin was shown to be essentially non-inferior to placebo.















