Related Research Articles
Catalysis is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst. Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process regenerating the catalyst.
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.

In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (Ea) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term Activation Energy was introduced in 1889 by the Swedish scientist Svante Arrhenius.

In biology, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate and residues that catalyse a reaction of that substrate. Although the active site occupies only ~10–20% of the volume of an enzyme, it is the most important part as it directly catalyzes the chemical reaction. It usually consists of three to four amino acids, while other amino acids within the protein are required to maintain the tertiary structure of the enzymes.

Aspartate carbamoyltransferase catalyzes the first step in the pyrimidine biosynthetic pathway.

In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may include other proteins, enzyme substrates, second messengers, hormones, or allosteric modulators. The binding event is often, but not always, accompanied by a conformational change that alters the protein's function. Binding to protein binding sites is most often reversible, but can also be covalent reversible or irreversible.

The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger ‡ symbol.
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.
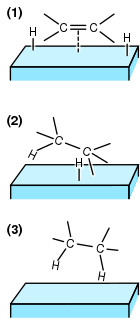
In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.

An oxyanion hole is a pocket in the active site of an enzyme that stabilizes transition state negative charge on a deprotonated oxygen or alkoxide. The pocket typically consists of backbone amides or positively charged residues. Stabilising the transition state lowers the activation energy necessary for the reaction, and so promotes catalysis. For example, proteases such as chymotrypsin contain an oxyanion hole to stabilise the tetrahedral intermediate anion formed during proteolysis and protects substrate's negatively charged oxygen from water molecules. Additionally, it may allow for insertion or positioning of a substrate, which would suffer from steric hindrance if it could not occupy the hole. Enzymes that catalyse multi-step reactions can have multiple oxyanion holes that stabilise different transition states in the reaction.
In biochemistry, avidity refers to the accumulated strength of multiple affinities of individual non-covalent binding interactions, such as between a protein receptor and its ligand, and is commonly referred to as functional affinity. Avidity differs from affinity, which describes the strength of a single interaction. However, because individual binding events increase the likelihood of occurrence of other interactions, avidity should not be thought of as the mere sum of its constituent affinities but as the combined effect of all affinities participating in the biomolecular interaction. A particular important aspect relates to the phenomenon of 'avidity entropy'. Biomolecules often form heterogenous complexes or homogeneous oligomers and multimers or polymers. If clustered proteins form an organized matrix, such as the clathrin-coat, the interaction is described as a matricity.

Enzyme catalysis is the increase in the rate of a process by a biological molecule, an "enzyme". Most enzymes are proteins, and most such processes are chemical reactions. Within the enzyme, generally catalysis occurs at a localized site, called the active site.

Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.

In enzymology, chorismate mutase is an enzyme that catalyzes the chemical reaction for the conversion of chorismate to prephenate in the pathway to the production of phenylalanine and tyrosine, also known as the shikimate pathway. Hence, this enzyme has one substrate, chorismate, and one product, prephenate. Chorismate mutase is found at a branch point in the pathway. The enzyme channels the substrate, chorismate to the biosynthesis of tyrosine and phenylalanine and away from tryptophan. Its role in maintaining the balance of these aromatic amino acids in the cell is vital. This is the single known example of a naturally occurring enzyme catalyzing a pericyclic reaction. Chorismate mutase is only found in fungi, bacteria, and higher plants. Some varieties of this protein may use the morpheein model of allosteric regulation.
In complexation catalysis, the term passive binding refers to any stabilizing interaction that is equally strong at the transition state level and in the reactant-catalyst complex.
In chemistry, solvent effects are the influence of a solvent on chemical reactivity or molecular associations. Solvents can have an effect on solubility, stability and reaction rates and choosing the appropriate solvent allows for thermodynamic and kinetic control over a chemical reaction.

In chemistry, a catalyst support is the material, usually a solid with a high surface area, to which a catalyst is affixed. The activity of heterogeneous catalysts is mainly promoted by atoms present at the accessible surface of the material. Consequently, great effort is made to maximize the specific surface area of a catalyst. One popular method for increasing surface area involves distributing the catalyst over the surface of the support. The support may be inert or participate in the catalytic reactions. Typical supports include various kinds of carbon, alumina, and silica.

Hydrogen-bond catalysis is a type of organocatalysis that relies on use of hydrogen bonding interactions to accelerate and control organic reactions. In biological systems, hydrogen bonding plays a key role in many enzymatic reactions, both in orienting the substrate molecules and lowering barriers to reaction. However, chemists have only recently attempted to harness the power of using hydrogen bonds to perform catalysis, and the field is relatively undeveloped compared to research in Lewis acid catalysis.

Supramolecular catalysis is not a well-defined field but it generally refers to an application of supramolecular chemistry, especially molecular recognition and guest binding, toward catalysis. This field was originally inspired by enzymatic system which, unlike classical organic chemistry reactions, utilizes non-covalent interactions such as hydrogen bonding, cation-pi interaction, and hydrophobic forces to dramatically accelerate rate of reaction and/or allow highly selective reactions to occur. Because enzymes are structurally complex and difficult to modify, supramolecular catalysts offer a simpler model for studying factors involved in catalytic efficiency of the enzyme. Another goal that motivates this field is the development of efficient and practical catalysts that may or may not have an enzyme equivalent in nature.
In chemistry, catalytic resonance theory was developed to describe the kinetics of reaction acceleration using dynamic catalyst surfaces. Catalytic reactions occurring on surfaces that undergo variation in surface binding energy and/or entropy exhibit overall increase in reaction rate when the surface binding energy frequencies are comparable to the natural frequencies of the surface reaction, adsorption, and desorption.
References
- ↑ A.J. Kirby Angewandte Chemie International Edition 1996 35 706–724.