| |
| Names | |
|---|---|
| IUPAC name (1S,4R,4aR,14S,14aS,18Z)-6,8,11-trihydroxy-3-methoxy-1-methyl-7,12-dioxo-1,4,7,12,13,14-hexahydro-4a,14a-epoxy-4,14-hex[3]ene[1,5]diynonaphtho[2,3-c]phenanthridine-2-carboxylic acid | |
| Other names (2R,4S,5S,8R,11Z,15S)-21,24,28-trihydroxy-7-methoxy-5-methyl-19,26-dioxo-3-oxa-16-azaheptacyclo[15.12.0.02,4.02,8.04,15.018,27.020,25]nonacosa-1(29),6,11,17,20,22,24,27-octaen-9,13-diyne-6-carboxylic acid | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| UNII | |
| |
| |
| Properties | |
| C30H19NO9 | |
| Molar mass | 537.473 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
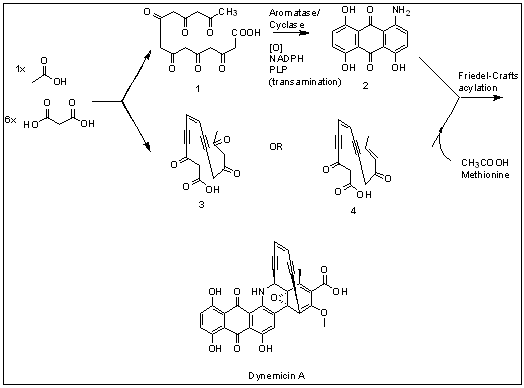
Dynemicin A is an anti-cancer enediyne drug. It displays properties which illustrate promise for cancer treatments, but still requires further research.