 | |
 | |
| Names | |
|---|---|
| IUPAC name (E)-octadec-9-enoic acid | |
| Other names (E)-9-octadecenoic acid (9E)-octadecenoic acid trans-9-octadecenoic acid 18:1 trans-9 C18:1 trans-9 | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.642 |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C 18H 34O 2 | |
| Molar mass | 282.46 g/mol |
| Appearance | colorless waxy solid |
| Density | 0.8734 g/cm3 |
| Melting point | 45 °C (113 °F) |
| −204.8·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Elaidic acid is a chemical compound with the formula C
18H
34O
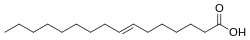
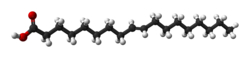
2, specifically the fatty acid with structural formula HOOC−(CH2)7−CH=CH−(CH2)7−CH3, with the double bond (between carbon atoms 9 and 10) in trans configuration. It is a colorless solid. Its salts and esters are called elaidates.
Contents
Elaidic acid is an unsaturated trans fatty acid, with code C18:1 trans-9. This compound has attracted attention because it is a major trans fat found in hydrogenated vegetable oils, and trans fats have been implicated in heart disease. [1]
It is the trans isomer of oleic acid. The name of the elaidinization reaction comes from elaidic acid.