The cell is the basic structural and functional unit of all forms of life. Every cell consists of cytoplasm enclosed within a membrane; many cells contain organelles, each with a specific function. The term comes from the Latin word cellula meaning 'small room'. Most cells are only visible under a microscope. Cells emerged on Earth about 4 billion years ago. All cells are capable of replication, protein synthesis, and motility.

In cell biology, the cytoplasm describes all material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are the cytosol, the organelles, and various cytoplasmic inclusions. The cytoplasm is about 80% water and is usually colorless.
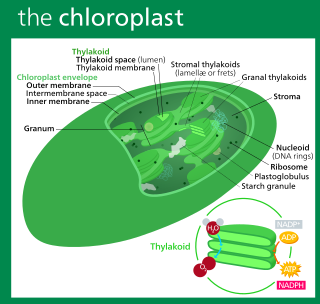
A chloroplast is a type of membrane-bound organelle known as a plastid that conducts photosynthesis mostly in plant and algal cells. The photosynthetic pigment chlorophyll captures the energy from sunlight, converts it, and stores it in the energy-storage molecules ATP and NADPH while freeing oxygen from water in the cells. The ATP and NADPH is then used to make organic molecules from carbon dioxide in a process known as the Calvin cycle. Chloroplasts carry out a number of other functions, including fatty acid synthesis, amino acid synthesis, and the immune response in plants. The number of chloroplasts per cell varies from one, in unicellular algae, up to 100 in plants like Arabidopsis and wheat.
In cell biology, an organelle is a specialized subunit, usually within a cell, that has a specific function. The name organelle comes from the idea that these structures are parts of cells, as organs are to the body, hence organelle, the suffix -elle being a diminutive. Organelles are either separately enclosed within their own lipid bilayers or are spatially distinct functional units without a surrounding lipid bilayer. Although most organelles are functional units within cells, some function units that extend outside of cells are often termed organelles, such as cilia, the flagellum and archaellum, and the trichocyst.
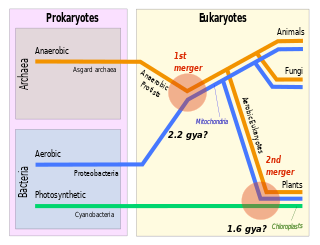
Symbiogenesis is the leading evolutionary theory of the origin of eukaryotic cells from prokaryotic organisms. The theory holds that mitochondria, plastids such as chloroplasts, and possibly other organelles of eukaryotic cells are descended from formerly free-living prokaryotes taken one inside the other in endosymbiosis. Mitochondria appear to be phylogenetically related to Rickettsiales bacteria, while chloroplasts are thought to be related to cyanobacteria.
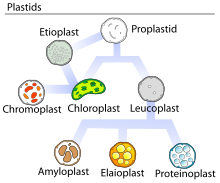
A plastid is a membrane-bound organelle found in the cells of plants, algae, and some other eukaryotic organisms. Plastids are considered to be intracellular endosymbiotic cyanobacteria.
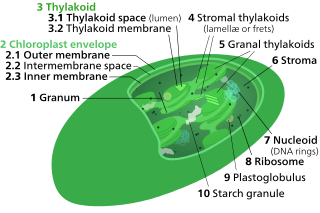
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as grana. Grana are connected by intergranal or stromal thylakoids, which join granum stacks together as a single functional compartment.

Chloroplasts contain several important membranes, vital for their function. Like mitochondria, chloroplasts have a double-membrane envelope, called the chloroplast envelope, but unlike mitochondria, chloroplasts also have internal membrane structures called thylakoids. Furthermore, one or two additional membranes may enclose chloroplasts in organisms that underwent secondary endosymbiosis, such as the euglenids and chlorarachniophytes.
Leucoplasts are a category of plastid and as such are organelles found in plant cells. They are non-pigmented, in contrast to other plastids such as the chloroplast.

Chromoplasts are plastids, heterogeneous organelles responsible for pigment synthesis and storage in specific photosynthetic eukaryotes. It is thought that like all other plastids including chloroplasts and leucoplasts they are descended from symbiotic prokaryotes.
A stromule is a microscopic structure found in plant cells. Stromules are highly dynamic structures extending from the surface of all plastid types, including proplastids, chloroplasts, etioplasts, leucoplasts, amyloplasts, and chromoplasts. Protrusions from and interconnections between plastids were observed in 1888 and 1908 and have been described sporadically in the literature since then. Stromules were recently rediscovered in 1997 and have since been reported to exist in a number of angiosperm species including Arabidopsis thaliana, wheat, rice and tomato, but their role is not yet fully understood.

A nuclear gene is a gene that has its DNA nucleotide sequence physically situated within the cell nucleus of a eukaryotic organism. This term is employed to differentiate nuclear genes, which are located in the cell nucleus, from genes that are found in mitochondria or chloroplasts. The vast majority of genes in eukaryotes are nuclear.

Proteinoplasts are specialized organelles found only in plant cells. Proteinoplasts belong to a broad category of organelles known as plastids. Plastids are specialized double-membrane organelles found in plant cells. Plastids perform a variety of functions such as metabolism of energy, and biological reactions. There are multiple types of plastids recognized including Leucoplasts, Chromoplasts, and Chloroplasts. Plastids are broken up into different categories based on characteristics such as size, function and physical traits. Chromoplasts help to synthesize and store large amounts of carotenoids. Chloroplasts are photosynthesizing structures that help to make light energy for the plant. Leucoplasts are a colorless type of plastid which means that no photosynthesis occurs here. The colorless pigmentation of the leucoplast is due to not containing the structural components of thylakoids unlike what is found in chloroplasts and chromoplasts that gives them their pigmentation. From leucoplasts stems the subtype, proteinoplasts, which contain proteins for storage. They contain crystalline bodies of protein and can be the sites of enzyme activity involving those proteins. Proteinoplasts are found in many seeds, such as brazil nuts, peanuts and pulses. Although all plastids contain high concentrations of protein, proteinoplasts were identified in the 1960s and 1970s as having large protein inclusions that are visible with both light microscopes and electron microscopes. Other subtypes of Leucoplasts include amyloplast, and elaioplasts. Amyloplasts help to store and synthesize starch molecules found in plants, while elaioplasts synthesize and store lipids in plant cells.
An apicoplast is a derived non-photosynthetic plastid found in most Apicomplexa, including Toxoplasma gondii, and Plasmodium falciparum and other Plasmodium spp., but not in others such as Cryptosporidium. It originated from algae through secondary endosymbiosis; there is debate as to whether this was a green or red alga. The apicoplast is surrounded by four membranes within the outermost part of the endomembrane system. The apicoplast hosts important metabolic pathways like fatty acid synthesis, isoprenoid precursor synthesis and parts of the heme biosynthetic pathway.
Oleosins are structural proteins found in vascular plant oil bodies and in plant cells. Oil bodies are not considered organelles because they have a single layer membrane and lack the pre-requisite double layer membrane in order to be considered an organelle. They are found in plant parts with high oil content that undergo extreme desiccation as part of their maturation process, and help stabilize the bodies.
The CoRR hypothesis states that the location of genetic information in cytoplasmic organelles permits regulation of its expression by the reduction-oxidation ("redox") state of its gene products.
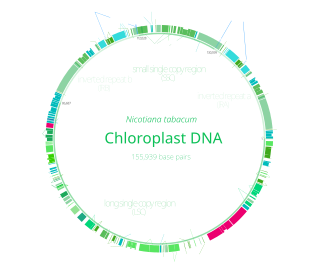
Chloroplast DNA (cpDNA) is the DNA located in chloroplasts, which are photosynthetic organelles located within the cells of some eukaryotic organisms. Chloroplasts, like other types of plastid, contain a genome separate from that in the cell nucleus. The existence of chloroplast DNA was identified biochemically in 1959, and confirmed by electron microscopy in 1962. The discoveries that the chloroplast contains ribosomes and performs protein synthesis revealed that the chloroplast is genetically semi-autonomous. The first complete chloroplast genome sequences were published in 1986, Nicotiana tabacum (tobacco) by Sugiura and colleagues and Marchantia polymorpha (liverwort) by Ozeki et al. Since then, a great number of chloroplast DNAs from various species have been sequenced.
A plastid is a membrane-bound organelle found in plants, algae and other eukaryotic organisms that contribute to the production of pigment molecules. Most plastids are photosynthetic, thus leading to color production and energy storage or production. There are many types of plastids in plants alone, but all plastids can be separated based on the number of times they have undergone endosymbiotic events. Currently there are three types of plastids; primary, secondary and tertiary. Endosymbiosis is reputed to have led to the evolution of eukaryotic organisms today, although the timeline is highly debated.
Rachel Leech was Professor of Plant Sciences at the University of York, UK. Her research focused on chloroplasts and she was a leader in the field of understanding their development and function. She was also one of the early adopters of Arabidopsis thaliana as a model plant to identify the genes involved in chloroplast division.