
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.

Baryte, barite or barytes ( or ) is a mineral consisting of barium sulfate (BaSO4). Baryte is generally white or colorless, and is the main source of the element barium. The baryte group consists of baryte, celestine (strontium sulfate), anglesite (lead sulfate), and anhydrite (calcium sulfate). Baryte and celestine form a solid solution (Ba,Sr)SO4.

Thermoluminescence dating (TL) is the determination, by means of measuring the accumulated radiation dose, of the time elapsed since material containing crystalline minerals was either heated or exposed to sunlight (sediments). As a crystalline material is heated during measurements, the process of thermoluminescence starts. Thermoluminescence emits a weak light signal that is proportional to the radiation dose absorbed by the material. It is a type of luminescence dating.
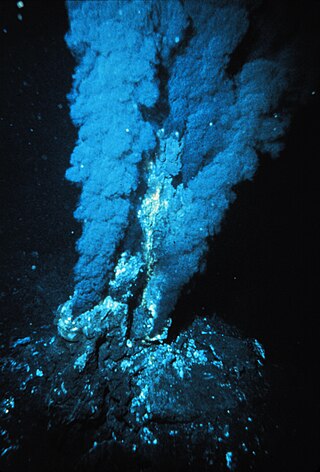
Hydrothermal vents are fissures on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hotspots. The dispersal of hydrothermal fluids throughout the global ocean at active vent sites creates hydrothermal plumes. Hydrothermal deposits are rocks and mineral ore deposits formed by the action of hydrothermal vents.
In physics, optically stimulated luminescence (OSL) is a method for measuring doses from ionizing radiation. It is used in at least two applications:
Microwave spectroscopy is the spectroscopy method that employs microwaves, i.e. electromagnetic radiation at GHz frequencies, for the study of matter.

Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.
There are 20 isotopes of sodium (11Na), ranging from 17
Na to 39
Na, and two isomers. 23
Na is the only stable isotope. It is considered a monoisotopic element and it has a standard atomic weight of 22.98976928(2). Sodium has two radioactive cosmogenic isotopes. With the exception of those two isotopes, all other isotopes have half-lives under a minute, most under a second. The shortest-lived is the unbound 18
Na, with a half-life of 1.3(4)×10−21 seconds.
Absolute dating is the process of determining an age on a specified chronology in archaeology and geology. Some scientists prefer the terms chronometric or calendar dating, as use of the word "absolute" implies an unwarranted certainty of accuracy. Absolute dating provides a numerical age or range, in contrast with relative dating, which places events in order without any measure of the age between events.
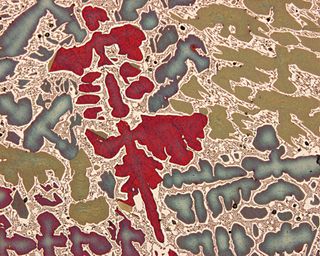
Characterization, when used in materials science, refers to the broad and general process by which a material's structure and properties are probed and measured. It is a fundamental process in the field of materials science, without which no scientific understanding of engineering materials could be ascertained. The scope of the term often differs; some definitions limit the term's use to techniques which study the microscopic structure and properties of materials, while others use the term to refer to any materials analysis process including macroscopic techniques such as mechanical testing, thermal analysis and density calculation. The scale of the structures observed in materials characterization ranges from angstroms, such as in the imaging of individual atoms and chemical bonds, up to centimeters, such as in the imaging of coarse grain structures in metals.
Luminescence dating refers to a group of chronological dating methods of determining how long ago mineral grains were last exposed to sunlight or sufficient heating. It is useful to geologists and archaeologists who want to know when such an event occurred. It uses various methods to stimulate and measure luminescence.
Potassium-40 (40K) is a radioactive isotope of potassium which has a long half-life of 1.25 billion years. It makes up about 0.012% of the total amount of potassium found in nature.

A gamma ray, also known as gamma radiation (symbol
γ
), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (3×1019 Hz) and wavelength less than 10 picometer (1×10−11 m) gamma ray photons have the highest photon energy of any form of electromagnetic radiation. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation gamma rays based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power.
In radiometric dating, closure temperature or blocking temperature refers to the temperature of a system, such as a mineral, at the time given by its radiometric date. In physical terms, the closure temperature is the temperature at which a system has cooled so that there is no longer any significant diffusion of the parent or daughter isotopes out of the system and into the external environment. The concept's initial mathematical formulation was presented in a seminal paper by Martin H. Dodson, "Closure temperature in cooling geochronological and petrological systems" in the journal Contributions to Mineralogy and Petrology, 1973, with refinements to a usable experimental formulation by other scientists in later years. This temperature varies broadly among different minerals and also differs depending on the parent and daughter atoms being considered. It is specific to a particular material and isotopic system.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI). The original application of NMR to condensed matter physics is nowadays mostly devoted to strongly correlated electron systems. It reveals large many-body couplings by fast broadband detection and it should not to be confused with solid state NMR, which aims at removing the effect of the same couplings by Magic Angle Spinning techniques.
Electron nuclear double resonance (ENDOR) is a magnetic resonance technique for elucidating the molecular and electronic structure of paramagnetic species. The technique was first introduced to resolve interactions in electron paramagnetic resonance (EPR) spectra. It is currently practiced in a variety of modalities, mainly in the areas of biophysics and heterogeneous catalysis.

The Philippine Nuclear Research Institute (PNRI) is a government agency under the Department of Science and Technology mandated to undertake research and development activities in the peaceful uses of nuclear energy, institute regulations on the said uses, and carry out the enforcement of said regulations to protect the health and safety of radiation workers and the general public.

Optically stimulated luminescence (OSL) thermochronometry is a dating method used to determine the time since quartz and/or feldspar began to store charge as it cools through the effective closure temperature. The closure temperature for quartz and Na-rich K-feldspar is 30-35 °C and 25 °C respectively. When quartz and feldspar are beneath the earth, they are hot. They cool when any geological process e.g. focused erosion causes their exhumation to the earth surface. As they cool, they trap electron charges originating from within the crystal lattice. These charges are accommodated within crystallographic defects or vacancies in their crystal lattices as the mineral cools below the closure temperature.
Hilary Stevenson was a food scientist and professor from Northern Ireland who made significant scientific contributions to the study of food irradiation in the 1980s and 1990s.











