
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.

Carbon monoxide is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest carbon oxide. In coordination complexes, the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry.

The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. The German chemists Fritz Haber and Carl Bosch developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using an iron metal catalyst under high temperatures and pressures. This reaction is slightly exothermic (i.e. it releases energy), meaning that the reaction is favoured at lower temperatures and higher pressures. It decreases entropy, complicating the process. Hydrogen is produced via steam reforming, followed by an iterative closed cycle to react hydrogen with nitrogen to produce ammonia.

Producer gas is fuel gas that is manufactured by blowing through a coke or coal fire with air and steam simultaneously. It mainly consists of carbon monoxide (CO), hydrogen (H2), as well as substantial amounts of nitrogen (N2). The caloric value of the producer gas is low (mainly because of its high nitrogen content), and the technology is obsolete. Improvements over producer gas, also obsolete, include water gas where the solid fuel is treated intermittently with air and steam and, far more efficiently synthesis gas where the solid fuel is replaced with methane.
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII.

Brazing is a metal-joining process in which two or more metal items are joined by melting and flowing a filler metal into the joint, with the filler metal having a lower melting point than the adjoining metal.
A reducing atmosphere is an atmospheric condition in which oxidation is prevented by absence of oxygen and other oxidizing gases or vapours, and which may contain actively reductant gases such as hydrogen, carbon monoxide, methane and hydrogen sulfide that would be readily oxidized to remove any free oxygen. Although Early Earth had had a reducing prebiotic atmosphere prior to the Proterozoic eon, starting at about 2.5 billion years ago in the late Neoarchaean period, the Earth's atmosphere experienced a significant rise in oxygen transitioned to an oxidizing atmosphere with a surplus of molecular oxygen (dioxygen, O2) as the primary oxidizing agent.

Industrial processes are procedures involving chemical, physical, electrical, or mechanical steps to aid in the manufacturing of an item or items, usually carried out on a very large scale. Industrial processes are the key components of heavy industry.

Carburizing, or carburising, is a heat treatment process in which iron or steel absorbs carbon while the metal is heated in the presence of a carbon-bearing material, such as charcoal or carbon monoxide. The intent is to make the metal harder and more wear resistant. Depending on the amount of time and temperature, the affected area can vary in carbon content. Longer carburizing times and higher temperatures typically increase the depth of carbon diffusion. When the iron or steel is cooled rapidly by quenching, the higher carbon content on the outer surface becomes hard due to the transformation from austenite to martensite, while the core remains soft and tough as a ferritic and/or pearlite microstructure.
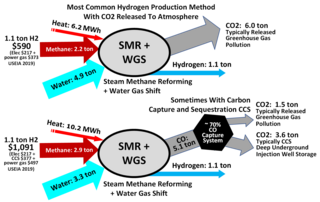
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen production. The reaction is represented by this equilibrium:

Case-hardening or Carburization is the process of introducing carbon to the surface of a low carbon iron or much more commonly low carbon steel object in order to enable the surface to be hardened.

Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases, as from a fireplace, oven, furnace, boiler or steam generator. It often refers to the exhaust gas of combustion at power plants. Technology is available to remove pollutants from flue gas at power plants.
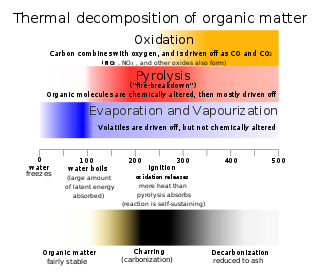
Thermal decomposition is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction.
Decarburization is the process of decreasing carbon content, which is the opposite of carburization.

Carbonitriding is a metallurgical surface modification technique that is used to increase the surface hardness of a metal, thereby reducing wear.
Water gas is a kind of fuel gas, a mixture of carbon monoxide and hydrogen. It is produced by "alternately hot blowing a fuel layer [coke] with air and gasifying it with steam". The caloric yield of this is about 10% of a modern syngas plant. Further making this technology unattractive, its precursor coke is expensive, whereas syngas uses cheaper precursor, mainly methane from natural gas.

Nitriding is a heat treating process that diffuses nitrogen into the surface of a metal to create a case-hardened surface. These processes are most commonly used on low-alloy steels. They are also used on titanium, aluminium and molybdenum.
A methane reformer is a device based on steam reforming, autothermal reforming or partial oxidation and is a type of chemical synthesis which can produce pure hydrogen gas from methane using a catalyst. There are multiple types of reformers in development but the most common in industry are autothermal reforming (ATR) and steam methane reforming (SMR). Most methods work by exposing methane to a catalyst at high temperature and pressure.
Forming gas is a mixture of hydrogen (mole fraction varies) and nitrogen. It is sometimes called a "dissociated ammonia atmosphere" due to the reaction which generates it:
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.











