Nanotechnology, often shortened to nanotech, is the use of matter on atomic, molecular, and supramolecular scales for industrial purposes. The earliest, widespread description of nanotechnology referred to the particular technological goal of precisely manipulating atoms and molecules for fabrication of macroscale products, also now referred to as molecular nanotechnology. A more generalized description of nanotechnology was subsequently established by the National Nanotechnology Initiative, which defined nanotechnology as the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). This definition reflects the fact that quantum mechanical effects are important at this quantum-realm scale, and so the definition shifted from a particular technological goal to a research category inclusive of all types of research and technologies that deal with the special properties of matter which occur below the given size threshold. It is therefore common to see the plural form "nanotechnologies" as well as "nanoscale technologies" to refer to the broad range of research and applications whose common trait is size.

Nanomaterials describe, in principle, materials of which a single unit is sized between 1 and 100 nm.

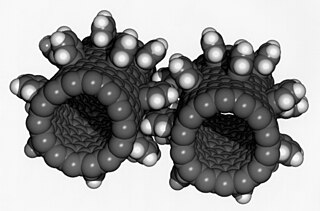
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At the lowest range, metal particles smaller than 1 nm are usually called atom clusters instead.
Nanomaterial-based catalysts are usually heterogeneous catalysts broken up into metal nanoparticles in order to enhance the catalytic process. Metal nanoparticles have high surface area, which can increase catalytic activity. Nanoparticle catalysts can be easily separated and recycled. They are typically used under mild conditions to prevent decomposition of the nanoparticles.

Nanobatteries are fabricated batteries employing technology at the nanoscale, particles that measure less than 100 nanometers or 10−7 meters. These batteries may be nano in size or may use nanotechnology in a macro scale battery. Nanoscale batteries can be combined to function as a macrobattery such as within a nanopore battery.
Green nanotechnology refers to the use of nanotechnology to enhance the environmental sustainability of processes producing negative externalities. It also refers to the use of the products of nanotechnology to enhance sustainability. It includes making green nano-products and using nano-products in support of sustainability.

Graphite oxide (GO), formerly called graphitic oxide or graphitic acid, is a compound of carbon, oxygen, and hydrogen in variable ratios, obtained by treating graphite with strong oxidizers and acids for resolving of extra metals. The maximally oxidized bulk product is a yellow solid with C:O ratio between 2.1 and 2.9, that retains the layer structure of graphite but with a much larger and irregular spacing.

An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells.
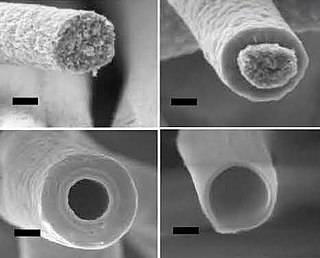
Silicon nanotubes are nanoparticles which create a tube-like structure from silicon atoms. As with silicon nanowires, they are technologically important due to their unusual physical properties, which differ fundamentally to those of bulk silicon. The first reports on silicon nanotubes appeared around the year 2000.
A nanofluid is a fluid containing nanometer-sized particles, called nanoparticles. These fluids are engineered colloidal suspensions of nanoparticles in a base fluid. The nanoparticles used in nanofluids are typically made of metals, oxides, carbides, or carbon nanotubes. Common base fluids include water, ethylene glycol and oil.

Rodney S. "Rod" Ruoff is an American physical chemist and nanoscience researcher. He is one of the world experts on carbon materials including carbon nanostructures such as fullerenes, nanotubes, graphene, diamond, and has had pioneering discoveries on such materials and others. Ruoff received his B.S. in chemistry from the University of Texas at Austin (1981) and his Ph.D. in chemical physics at the University of Illinois-Urbana (1988). After a Fulbright Fellowship at the MPI fuer Stroemungsforschung in Goettingen, Germany (1989) and postdoctoral work at the IBM T. J. Watson Research Center (1990–91), Ruoff became a staff scientist in the Molecular Physics Laboratory at SRI International (1991–1996). He is currently UNIST Distinguished Professor at the Ulsan National Institute of Science and Technology (UNIST), and the director of the Center for Multidimensional Carbon Materials, an Institute for Basic Science Center located at UNIST.
The applications of nanotechnology, commonly incorporate industrial, medicinal, and energy uses. These include more durable construction materials, therapeutic drug delivery, and higher density hydrogen fuel cells that are environmentally friendly. Being that nanoparticles and nanodevices are highly versatile through modification of their physiochemical properties, they have found uses in nanoscale electronics, cancer treatments, vaccines, hydrogen fuel cells, and nanographene batteries.

Nanoparticles are classified as having at least one of its dimensions in the range of 1-100 nanometers (nm). The small size of nanoparticles allows them to have unique characteristics which may not be possible on the macro-scale. Self-assembly is the spontaneous organization of smaller subunits to form larger, well-organized patterns. For nanoparticles, this spontaneous assembly is a consequence of interactions between the particles aimed at achieving a thermodynamic equilibrium and reducing the system’s free energy. The thermodynamics definition of self-assembly was introduced by Professor Nicholas A. Kotov. He describes self-assembly as a process where components of the system acquire non-random spatial distribution with respect to each other and the boundaries of the system. This definition allows one to account for mass and energy fluxes taking place in the self-assembly processes.
Potential graphene applications include lightweight, thin, and flexible electric/photonics circuits, solar cells, and various medical, chemical and industrial processes enhanced or enabled by the use of new graphene materials.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and cost.

In materials and electric battery research, cobalt oxide nanoparticles usually refers to particles of cobalt(II,III) oxide Co
3O
4 of nanometer size, with various shapes and crystal structures.

Irshad Hussain is a Pakistani Scientist in the field of chemistry and among the few pioneers to initiate nanomaterials research in Pakistan.
There are many water purifiers available in the market which use different techniques like boiling, filtration, distillation, chlorination, sedimentation and oxidation. Currently nanotechnology plays a vital role in water purification techniques. Nanotechnology is the process of manipulating atoms on a nanoscale. In nanotechnology, nanomembranes are used with the purpose of softening the water and removal of contaminants such as physical, biological and chemical contaminants. There are variety of techniques in nanotechnology which uses nanoparticles for providing safe drinking water with a high level of effectiveness. Some techniques have become commercialized.
Lianzhou Wang is a Chinese Australian materials scientist and professor in the School of Chemical Engineering at the University of Queensland. He is director of the Nanomaterials Centre (Nanomac) and a senior group member at the Australian Institute for Bioengineering and Nanotechnology at the University of Queensland, as well as a Fellow of the Royal Society of Chemistry.
Ramakrishna Podila is an Indian-born American physicist and nanomaterials researcher. He is currently an Associate Professor of Physics in the Department of Physics and Astronomy at Clemson University and is the director of the Clemson Nano-bio lab. He is known for his interdisciplinary research at the interface of physics, biology, and nanoscience. His lab integrates the principles of condensed matter physics, optical spectroscopy, and physiological chemistry to understand physics at the nanoscale and nano-bio interfaces.









