
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO4·2H2O. It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywall. Alabaster, a fine-grained white or lightly tinted variety of gypsum, has been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite.

Brine is a high-concentration solution of salt in water. In diverse contexts, brine may refer to the salt solutions ranging from about 3.5% up to about 26%. Brine forms naturally due to evaporation of ground saline water but it is also generated in the mining of sodium chloride. Brine is used for food processing and cooking, for de-icing of roads and other structures, and in a number of technological processes. It is also a by-product of many industrial processes, such as desalination, so it requires wastewater treatment for proper disposal or further utilization.

An evaporite is a water-soluble sedimentary mineral deposit that results from concentration and crystallization by evaporation from an aqueous solution. There are two types of evaporite deposits: marine, which can also be described as ocean deposits, and non-marine, which are found in standing bodies of water such as lakes. Evaporites are considered sedimentary rocks and are formed by chemical sediments.
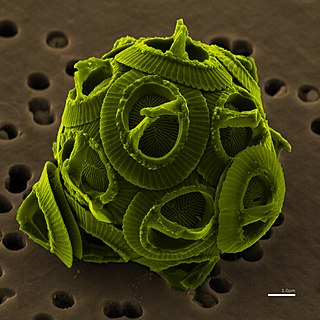
Geomicrobiology is the scientific field at the intersection of geology and microbiology and is a major subfield of geobiology. It concerns the role of microbes on geological and geochemical processes and effects of minerals and metals to microbial growth, activity and survival. Such interactions occur in the geosphere, the atmosphere and the hydrosphere. Geomicrobiology studies microorganisms that are driving the Earth's biogeochemical cycles, mediating mineral precipitation and dissolution, and sorbing and concentrating metals. The applications include for example bioremediation, mining, climate change mitigation and public drinking water supplies.

Halite, commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride (NaCl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pink, red, orange, yellow or gray depending on inclusion of other materials, impurities, and structural or isotopic abnormalities in the crystals. It commonly occurs with other evaporite deposit minerals such as several of the sulfates, halides, and borates. The name halite is derived from the Ancient Greek word for "salt", ἅλς (háls).

Anhydrite, or anhydrous calcium sulfate, is a mineral with the chemical formula CaSO4. It is in the orthorhombic crystal system, with three directions of perfect cleavage parallel to the three planes of symmetry. It is not isomorphous with the orthorhombic barium (baryte) and strontium (celestine) sulfates, as might be expected from the chemical formulas. Distinctly developed crystals are somewhat rare, the mineral usually presenting the form of cleavage masses. The Mohs hardness is 3.5, and the specific gravity is 2.9. The color is white, sometimes greyish, bluish, or purple. On the best developed of the three cleavages, the lustre is pearly; on other surfaces it is glassy. When exposed to water, anhydrite readily transforms to the more commonly occurring gypsum, (CaSO4·2H2O) by the absorption of water. This transformation is reversible, with gypsum or calcium sulfate hemihydrate forming anhydrite by heating to around 200 °C (400 °F) under normal atmospheric conditions. Anhydrite is commonly associated with calcite, halite, and sulfides such as galena, chalcopyrite, molybdenite, and pyrite in vein deposits.

Dolomite (also known as dolomite rock, dolostone or dolomitic rock) is a sedimentary carbonate rock that contains a high percentage of the mineral dolomite, CaMg(CO3)2. It occurs widely, often in association with limestone and evaporites, though it is less abundant than limestone and rare in Cenozoic rock beds (beds less than about 66 million years in age). The first geologist to distinguish dolomite rock from limestone was Belsazar Hacquet in 1778.
The pedosphere is the outermost layer of the Earth that is composed of soil and subject to soil formation processes. It exists at the interface of the lithosphere, atmosphere, hydrosphere and biosphere. The pedosphere is the skin of the Earth and only develops when there is a dynamic interaction between the atmosphere, biosphere, lithosphere and the hydrosphere. The pedosphere is the foundation of terrestrial life on Earth.

Searles Lake is an endorheic dry lake in the Searles Valley of the Mojave Desert, in northwestern San Bernardino County, California. The lake in the past was also called Slate Range Lake and Borax Lake.

Carnallite (also carnalite) is an evaporite mineral, a hydrated potassium magnesium chloride with formula KCl.MgCl2·6(H2O). It is variably colored yellow to white, reddish, and sometimes colorless or blue. It is usually massive to fibrous with rare pseudohexagonal orthorhombic crystals. The mineral is deliquescent (absorbs moisture from the surrounding air) and specimens must be stored in an airtight container.

The Red Hills, also referred to as Gypsum Hills, is the name of a physiographic region located mostly in Clark, Comanche and Barber counties in southern and central Kansas. This undulating terrain of red-tinted sediments, a product of the underlying geology, does not fit the conventional description of the Great Plains landscape of Kansas.

Bristol Lake is a dry lake in the Mojave Desert of San Bernardino County, California, 42 km (26 mi) northeast of Twentynine Palms.

A sabkha is a coastal, supratidal mudflat or sandflat in which evaporite-saline minerals accumulate as the result of semiarid to arid climate. Sabkhas are gradational between land and intertidal zone within restricted coastal plains just above normal high-tide level. Within a sabkha, evaporite-saline minerals sediments typically accumulate below the surface of mudflats or sandflats. Evaporite-saline minerals, tidal-flood, and aeolian deposits characterize many sabkhas found along modern coastlines. The accepted type locality for a sabkha is at the southern coast of the Persian Gulf, in the United Arab Emirates. Sabkha is a phonetic transliteration of the Arabic word used to describe any form of salt flat. A sabkha is also known as a sabkhah,sebkha, or coastal sabkha.

Hutt Lagoon is a marine salt lake located near the Indian Ocean coast 2 kilometres (1.2 mi) north of the mouth of the Hutt River, in the Mid West region of Western Australia.
The mineralogy of Mars is the chemical composition of rocks and soil that encompass the surface of Mars. Various orbital crafts have used spectroscopic methods to identify the signature of some minerals. The planetary landers performed concrete chemical analysis of the soil in rocks to further identify and confirm the presence of other minerals. The only samples of Martian rocks that are on Earth are in the form of meteorites. The elemental and atmospheric composition along with planetary conditions is essential in knowing what minerals can be formed from these base parts.

Shallow water marine environment refers to the area between the shore and deeper water, such as a reef wall or a shelf break. This environment is characterized by oceanic, geological and biological conditions, as described below. The water in this environment is shallow and clear, allowing the formation of different sedimentary structures, carbonate rocks, coral reefs, and allowing certain organisms to survive and become fossils.

Across the southern highlands of Mars, approximately 640 sites of chloride-bearing deposits have been identified using the Thermal Emission Imaging System (THEMIS). These isolated, irregularly shaped patches have been dated to the older geologic periods on Mars: Noachian and Hesperian periods. On Earth, chlorides are known to form through aqueous processes. Similar processes are expected to be responsible for the formation of chloride deposits on Mars. The finding of these deposits is significant in that it provides further evidence for the presence of surface or subsurface water in ancient Mars.

Leonite is a hydrated double sulfate of magnesium and potassium. It has the formula K2SO4·MgSO4·4H2O. The mineral was named after Leo Strippelmann, who was director of the salt works at Westeregeln in Germany. The mineral is part of the blodite group of hydrated double sulfate minerals.
Mars habitability analogue environments on Earth are environments that share potentially relevant astrobiological conditions with Mars. These include sites that are analogues of potential subsurface habitats, and deep subsurface habitats.

Salar Ignorado is a salar in the Andes of Chile's Atacama Region at 4,250 metres (13,940 ft) elevation. Located just south of Cerro Bayo volcano, it comprises 0.7 square kilometres (0.27 sq mi) of salt flats, sand dunes and numerous pools of open water. The waters of Salar Ignorado, unlike these of other salt flats in the central Andes, are acidic owing to the input of sulfuric acid from hydrothermal water and the weathering of volcanic rocks.

















