
An axon, or nerve fiber, is a long, slender projection of a nerve cell, or neuron, in vertebrates, that typically conducts electrical impulses known as action potentials away from the nerve cell body. The function of the axon is to transmit information to different neurons, muscles, and glands. In certain sensory neurons, such as those for touch and warmth, the axons are called afferent nerve fibers and the electrical impulse travels along these from the periphery to the cell body and from the cell body to the spinal cord along another branch of the same axon. Axon dysfunction has caused many inherited and acquired neurological disorders which can affect both the peripheral and central neurons. Nerve fibers are classed into three types – group A nerve fibers, group B nerve fibers, and group C nerve fibers. Groups A and B are myelinated, and group C are unmyelinated. These groups include both sensory fibers and motor fibers. Another classification groups only the sensory fibers as Type I, Type II, Type III, and Type IV.

A nerve is an enclosed, cable-like bundle of nerve fibers in the peripheral nervous system.
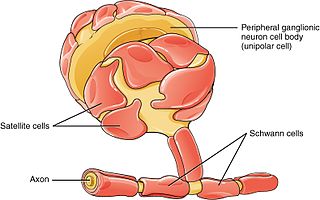
Schwann cells or neurolemmocytes are the principal glia of the peripheral nervous system (PNS). Glial cells function to support neurons and in the PNS, also include satellite cells, olfactory ensheathing cells, enteric glia and glia that reside at sensory nerve endings, such as the Pacinian corpuscle. The two types of Schwann cells are myelinating and nonmyelinating. Myelinating Schwann cells wrap around axons of motor and sensory neurons to form the myelin sheath. The Schwann cell promoter is present in the downstream region of the human dystrophin gene that gives shortened transcript that are again synthesized in a tissue-specific manner.

A motor nerve is a nerve located in the central nervous system (CNS), usually the spinal cord, that sends motor signals from the CNS to the muscles of the body. This is different from the motor neuron, which includes a cell body and branching of dendrites, while the nerve is made up of a bundle of axons. Motor nerves act as efferent nerves which carry information out from the CNS to muscles, as opposed to afferent nerves, which send signals from sensory receptors in the periphery to the CNS. Efferent nerves can also connect to glands or other organs/issues instead of muscles. In addition, there are nerves that serve as both sensory and motor nerves called mixed nerves.

Wallerian degeneration is an active process of degeneration that results when a nerve fiber is cut or crushed and the part of the axon distal to the injury degenerates. A related process of dying back or retrograde degeneration known as 'Wallerian-like degeneration' occurs in many neurodegenerative diseases, especially those where axonal transport is impaired such as ALS and Alzheimer's disease. Primary culture studies suggest that a failure to deliver sufficient quantities of the essential axonal protein NMNAT2 is a key initiating event.
Neurotmesis is part of Seddon's classification scheme used to classify nerve damage. It is the most serious nerve injury in the scheme. In this type of injury, both the nerve and the nerve sheath are disrupted. While partial recovery may occur, complete recovery is impossible.
Axonotmesis is an injury to the peripheral nerve of one of the extremities of the body. The axons and their myelin sheath are damaged in this kind of injury, but the endoneurium, perineurium and epineurium remain intact. Motor and sensory functions distal to the point of injury are completely lost over time leading to Wallerian degeneration due to ischemia, or loss of blood supply. Axonotmesis is usually the result of a more severe crush or contusion than neurapraxia.
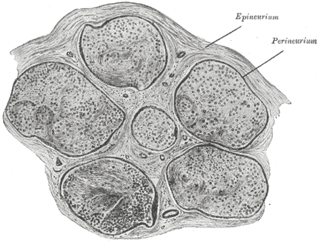
The epineurium is the outermost layer of dense irregular connective tissue surrounding a peripheral nerve. It usually surrounds multiple nerve fascicles as well as blood vessels which supply the nerve. Smaller branches of these blood vessels penetrate into the perineurium. In addition to blood vessels which supply the nerve, lymphocytes and fibroblasts are also present and contribute to the production of collagen fibers that form the backbone of the epineurium. In addition to providing structural support, lymphocytes and fibroblasts also play a vital role in maintenance and repair of the surrounding tissues.
Neural engineering is a discipline within biomedical engineering that uses engineering techniques to understand, repair, replace, or enhance neural systems. Neural engineers are uniquely qualified to solve design problems at the interface of living neural tissue and non-living constructs.

The endoneurium is a layer of delicate connective tissue around the myelin sheath of each myelinated nerve fiber in the peripheral nervous system. Its component cells are called endoneurial cells. The endoneuria with their enclosed nerve fibers are bundled into groups called nerve fascicles, each fascicle within its own protective sheath called a perineurium. In sufficiently large nerves multiple fascicles, each with its blood supply and fatty tissue, may be bundled within yet another sheath, the epineurium.

Nerve injury is an injury to nervous tissue. There is no single classification system that can describe all the many variations of nerve injuries. In 1941, Seddon introduced a classification of nerve injuries based on three main types of nerve fiber injury and whether there is continuity of the nerve. Usually, however, peripheral nerve injuries are classified in five stages, based on the extent of damage to both the nerve and the surrounding connective tissue, since supporting glial cells may be involved.
Neuroregeneration refers to the regrowth or repair of nervous tissues, cells or cell products. Such mechanisms may include generation of new neurons, glia, axons, myelin, or synapses. Neuroregeneration differs between the peripheral nervous system (PNS) and the central nervous system (CNS) by the functional mechanisms involved, especially in the extent and speed of repair. When an axon is damaged, the distal segment undergoes Wallerian degeneration, losing its myelin sheath. The proximal segment can either die by apoptosis or undergo the chromatolytic reaction, which is an attempt at repair. In the CNS, synaptic stripping occurs as glial foot processes invade the dead synapse.
Neural tissue engineering is a specific sub-field of tissue engineering. Neural tissue engineering is primarily a search for strategies to eliminate inflammation and fibrosis upon implantation of foreign substances. Often foreign substances in the form of grafts and scaffolds are implanted to promote nerve regeneration and to repair damage caused to nerves of both the central nervous system (CNS) and peripheral nervous system (PNS) by an injury.

Glial scar formation (gliosis) is a reactive cellular process involving astrogliosis that occurs after injury to the central nervous system. As with scarring in other organs and tissues, the glial scar is the body's mechanism to protect and begin the healing process in the nervous system.

Classification of peripheral nerve injury assists in prognosis and determination of treatment strategy. Classification of nerve injury was described by Seddon in 1943 and by Sunderland in 1951. The lowest degree of nerve injury in which the nerve remains intact but signaling ability is damaged is called neurapraxia. The second degree in which the axon is damaged but the surrounding connecting tissue remains intact is called axonotmesis. The last degree in which both the axon and connective tissue are damaged is called neurotmesis.

Olfactory ensheathing cells (OECs), also known as olfactory ensheathing glia or olfactory ensheathing glial cells, are a type of macroglia found in the nervous system. They are also known as olfactory Schwann cells, because they ensheath the non-myelinated axons of olfactory neurons in a similar way to which Schwann cells ensheath non-myelinated peripheral neurons. They also share the property of assisting axonal regeneration.
Nerve allotransplantation is the transplantation of a nerve to a receiver from a donor of the same species. For example, nerve tissue is transplanted from one person to another. Allotransplantation is a commonly used type of transplantation of which nerve repair is one specific aspect.
Cryoneurolysis, also referred to as cryoanalgesia, is a medical procedure that temporarily blocks nerve conduction along peripheral nerve pathways. The procedure, which inserts a small probe to freeze the target nerve, can facilitate complete regeneration of the structure and function of the affected nerve. Cryoneurolysis has been used to treat a variety of painful conditions.
Preferential motor reinnervation (PMR) refers to the tendency of a regenerating axon in the peripheral nervous system (PNS) to reinnervate a motor pathway as opposed to a somatosensory pathway. PMR affects how nerves regenerate and reinnervate within the PNS after surgical procedures or traumatic injuries. It is important to understand in order to further develop axonal regrowth surgical techniques. Further research of preferential motor reinnervation will lead to a better understanding of peripheral nervous system function in the human body regarding cell roles and abilities.
Spinal cord injury research seeks new ways to cure or treat spinal cord injury in order to lessen the debilitating effects of the injury in the short or long term. There is no cure for SCI, and current treatments are mostly focused on spinal cord injury rehabilitation and management of the secondary effects of the condition. Two major areas of research include neuroprotection, ways to prevent damage to cells caused by biological processes that take place in the body after the insult, and neuroregeneration, regrowing or replacing damaged neural circuits.












