
Polycystic ovary syndrome, or polycystic ovarian syndrome (PCOS), is the most common endocrine disorder in women of reproductive age. The syndrome is named after cysts which form on the ovaries of some people with this condition, though this is not a universal symptom, and not the underlying cause of the disorder.

Metabolic syndrome is a clustering of at least three of the following five medical conditions: abdominal obesity, high blood pressure, high blood sugar, high serum triglycerides, and low serum high-density lipoprotein (HDL).
Insulin resistance (IR) is a pathological condition in which cells either fail to respond normally to the hormone insulin or downregulate insulin receptors in response to hyperinsulinemia.

The glucose tolerance test is a medical test in which glucose is given and blood samples taken afterward to determine how quickly it is cleared from the blood. The test is usually used to test for diabetes, insulin resistance, impaired beta cell function, and sometimes reactive hypoglycemia and acromegaly, or rarer disorders of carbohydrate metabolism. In the most commonly performed version of the test, an oral glucose tolerance test (OGTT), a standard dose of glucose is ingested by mouth and blood levels are checked two hours later. Many variations of the GTT have been devised over the years for various purposes, with different standard doses of glucose, different routes of administration, different intervals and durations of sampling, and various substances measured in addition to blood glucose.
Hyperglycemia is a condition in which an excessive amount of glucose circulates in the blood plasma. This is generally a blood sugar level higher than 11.1 mmol/L (200 mg/dL), but symptoms may not start to become noticeable until even higher values such as 13.9–16.7 mmol/L (~250–300 mg/dL). A subject with a consistent range between ~5.6 and ~7 mmol/L is considered slightly hyperglycemic, and above 7 mmol/L is generally held to have diabetes. For diabetics, glucose levels that are considered to be too hyperglycemic can vary from person to person, mainly due to the person's renal threshold of glucose and overall glucose tolerance. On average, however, chronic levels above 10–12 mmol/L (180–216 mg/dL) can produce noticeable organ damage over time.

Ketosis is a metabolic state characterized by elevated levels of ketone bodies in the blood or urine. Physiological ketosis is a normal response to low glucose availability, such as low-carbohydrate diets or fasting, that provides an additional energy source for the brain in the form of ketones. In physiological ketosis, ketones in the blood are elevated above baseline levels, but the body's acid–base homeostasis is maintained. This contrasts with ketoacidosis, an uncontrolled production of ketones that occurs in pathologic states and causes a metabolic acidosis, which is a medical emergency. Ketoacidosis is most commonly the result of complete insulin deficiency in type 1 diabetes or late-stage type 2 diabetes. Ketone levels can be measured in blood, urine or breath and are generally between 0.5 and 3.0 millimolar (mM) in physiological ketosis, while ketoacidosis may cause blood concentrations greater than 10 mM.

Type 2 diabetes (T2D), formerly known as adult-onset diabetes, is a form of diabetes mellitus that is characterized by high blood sugar, insulin resistance, and relative lack of insulin. Common symptoms include increased thirst, frequent urination, fatigue and unexplained weight loss. Symptoms may also include increased hunger, having a sensation of pins and needles, and sores (wounds) that do not heal. Often symptoms come on slowly. Long-term complications from high blood sugar include heart disease, strokes, diabetic retinopathy which can result in blindness, kidney failure, and poor blood flow in the limbs which may lead to amputations. The sudden onset of hyperosmolar hyperglycemic state may occur; however, ketoacidosis is uncommon.
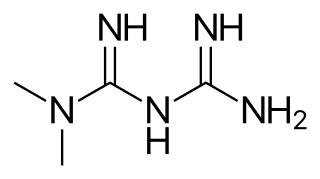
Metformin, sold under the brand name Glucophage, among others, is the main first-line medication for the treatment of type 2 diabetes, particularly in people who are overweight. It is also used in the treatment of polycystic ovary syndrome. It is sometimes used as an off-label adjunct to lessen the risk of metabolic syndrome in people who take antipsychotics. Metformin is not associated with weight gain and is taken by mouth.

The blood sugar level, blood sugar concentration, blood glucose level, or glycemia, is the measure of glucose concentrated in the blood. The body tightly regulates blood glucose levels as a part of metabolic homeostasis.

Gestational diabetes is a condition in which a woman without diabetes develops high blood sugar levels during pregnancy. Gestational diabetes generally results in few symptoms; however, it increases the risk of pre-eclampsia, depression, and of needing a Caesarean section. Babies born to mothers with poorly treated gestational diabetes are at increased risk of macrosomia, of having hypoglycemia after birth, and of jaundice. If untreated, diabetes can also result in stillbirth. Long term, children are at higher risk of being overweight and of developing type 2 diabetes.
Diabetes is a chronic disease in cats whereby either insufficient insulin response or insulin resistance leads to persistently high blood glucose concentrations. Diabetes affects up to 1 in 230 cats, and may be becoming increasingly common. Diabetes is less common in cats than in dogs. The condition is treatable, and if treated properly the cat can experience a normal life expectancy. In cats with type 2 diabetes, prompt effective treatment may lead to diabetic remission, in which the cat no longer needs injected insulin. Untreated, the condition leads to increasingly weak legs in cats and eventually to malnutrition, ketoacidosis and/or dehydration, and death.

Laminitis is a disease that affects the feet of ungulates and is found mostly in horses and cattle. Clinical signs include foot tenderness progressing to inability to walk, increased digital pulses, and increased temperature in the hooves. Severe cases with outwardly visible clinical signs are known by the colloquial term founder, and progression of the disease will lead to perforation of the coffin bone through the sole of the hoof or being unable to stand up, requiring euthanasia.

Hyperinsulinemia is a condition in which there are excess levels of insulin circulating in the blood relative to the level of glucose. While it is often mistaken for diabetes or hyperglycaemia, hyperinsulinemia can result from a variety of metabolic diseases and conditions, as well as non-nutritive sugars in the diet. While hyperinsulinemia is often seen in people with early stage type 2 diabetes mellitus, it is not the cause of the condition and is only one symptom of the disease. Type 1 diabetes only occurs when pancreatic beta-cell function is impaired. Hyperinsulinemia can be seen in a variety of conditions including diabetes mellitus type 2, in neonates and in drug-induced hyperinsulinemia. It can also occur in congenital hyperinsulinism, including nesidioblastosis.
Specific dynamic action (SDA), also known as thermic effect of food (TEF) or dietary induced thermogenesis (DIT), is the amount of energy expenditure above the basal metabolic rate due to the cost of processing food for use and storage. Heat production by brown adipose tissue which is activated after consumption of a meal is an additional component of dietary induced thermogenesis. The thermic effect of food is one of the components of metabolism along with resting metabolic rate and the exercise component. A commonly used estimate of the thermic effect of food is about 10% of one's caloric intake, though the effect varies substantially for different food components. For example, dietary fat is very easy to process and has very little thermic effect, while protein is hard to process and has a much larger thermic effect.

Isomaltulose is a disaccharide carbohydrate composed of glucose and fructose. It is naturally present in honey and sugarcane extracts and is also produced industrially from table sugar (sucrose) and used as a sugar alternative.
Equine polysaccharide storage myopathy is a hereditary glycogen storage disease of horses that causes exertional rhabdomyolysis. It is currently known to affect the following breeds American Quarter Horses, American Paint Horses, Warmbloods, Cobs, Dales Ponies, Thoroughbreds, Arabians, New Forest ponies, and a large number of Heavy horse breeds. While incurable, PSSM can be managed with appropriate diet and exercise. There are currently 2 subtypes, known as Type 1 PSSM and Type 2 PSSM.

An easy keeper, easy doer, or good doer is a horse that can live on relatively little feed. The opposite of an easy keeper is a hard keeper, an animal that is prone to be too thin and has difficulty maintaining adequate weight.

Pituitary pars intermedia dysfunction (PPID), or equine Cushing's disease, is an endocrine disease affecting the pituitary gland of horses. It is most commonly seen in older animals, and is classically associated with the formation of a long, wavy coat (hirsutism) and chronic laminitis.
Obesity is defined as an abnormal accumulation of body fat, usually 20% or more over an individual's ideal body weight. This is often described as a body mass index (BMI) over 30. However, BMI does not account for whether the excess weight is fat or muscle, and is not a measure of body composition. For most people, however, BMI is an indication used worldwide to estimate nutritional status. Obesity is usually the result of consuming more calories than the body needs and not expending that energy by doing exercise. There are genetic causes and hormonal disorders that cause people to gain significant amounts of weight but this is rare. People in the obese category are much more likely to suffer from fertility problems than people of normal healthy weight.

Serpin A12 is a glycoprotein that is a class A member of the serine protease inhibitor (serpin) family. In humans, Serpin A12 is encoded by the SERPINA12 gene.















