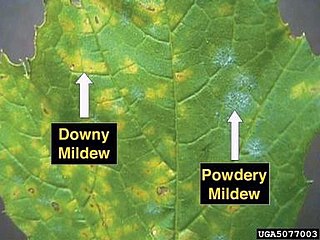
Powdery mildew is a fungal disease that affects a wide range of plants. Powdery mildew diseases are caused by many different species of ascomycete fungi in the order Erysiphales. Powdery mildew is one of the easier plant diseases to identify, as the signs of the causal pathogen are quite distinctive. Infected plants display white powdery spots on the leaves and stems. This mycelial layer may quickly spread to cover all of the leaves. The lower leaves are the most affected, but the mildew can appear on any above-ground part of the plant. As the disease progresses, the spots get larger and denser as large numbers of asexual spores are formed, and the mildew may spread up and down the length of the plant.

Uncinula necator is a fungus that causes powdery mildew of grape. It is a common pathogen of Vitis species, including the wine grape, Vitis vinifera. The fungus is believed to have originated in North America. European varieties of Vitis vinifera are more or less susceptible to this fungus. Uncinula necator infects all green tissue on the grapevine, including leaves and young berries. It can cause crop loss and poor wine quality if untreated. The sexual stage of this pathogen requires free moisture to release ascospores from its cleistothecia in the spring. However, free moisture is not needed for secondary spread via conidia; high atmospheric humidity is sufficient. Its anamorph is called Oidium tuckeri.

Erysiphales are an order of ascomycete fungi. The order contains one family, Erysiphaceae. Many of them cause plant diseases called powdery mildew.

Blumeria graminis is a fungus that causes powdery mildew on grasses, including cereals. It is the only species in the genus Blumeria. It has also been called Erysiphe graminis and Oidium monilioides or Oidium tritici.
Powdery mildew is a fungal disease of barley caused by Blumeria graminis f. sp. hordei. The disease has a worldwide distribution and is most damaging in cool, wet climates. The host range of the form species hordei is restricted to barley and other Hordeum species.

Hyaloperonospora parasitica is an oomycete from the family Peronosporaceae. It has been considered for a long time to cause downy mildew of a variety of species within the Brassicaceae, on which the disease can cause economically important damage by killing seedlings or affecting the quality of produce intended for freezing. Hyaloperonospora parasitica causes downy mildew on a wide range of many different plants. It belongs to the Kingdom Chromista, the phylum Oomycota, and the family Peronosporaceae. The former name for H. parasitica was Peronospora parasitica until it was reclassified and put in the genus Hyaloperonospora. It is an especially vicious disease on crops of the family Brassicaceae. It is most famous for being a model pathogen of Arabidopsis thaliana which is a model organism used for experimental purposes. Accordingly, the former Hyaloperonospora parasitica has been split into a large number of species. For instance, the taxonomically correct name of the parasite of the well-known model organism Arabidopsis thaliana is Hyaloperonospora arabidopsidis, not H. parasitica, whereas the pathogen of Brassica has to be called Hyaloperonospora brassicae.

Pseudocercosporella capsellae is a plant pathogen infecting crucifers. P. capsellae is the causal pathogen of white leaf spot disease, which is an economically significant disease in global agriculture. P. capsellae has a significant affect on crop yields on agricultural products, such as canola seed and rapeseed. Researchers are working hard to find effective methods of controlling this plant pathogen, using cultural control, genetic resistance, and chemical control practices. Due to its rapidly changing genome, P. capsellae is a rapidly emerging plant pathogen that is beginning to spread globally and affect farmers around the world.

Erysiphe betae is a fungal plant pathogen. It is a form of powdery mildew that can affect crops of sugar beet, that could cause up to a 30% yield loss. The fungus occurs worldwide in all regions where sugar beet is grown and it also infects other edible crops, e.g. beetroot.

Mycosphaerella brassicicola is a plant pathogen. The pathogen is the teleomorph phase of an ascomycete fungus, which causes the ring spot disease of brassicas. The supplementary anamorph phase Asteromella brassicae produces conidia through its asexual reproduction, however these spores are not confirmed to cause disease in host plants.

Leveillula taurica is an obligate fungal pathogen, from the phylum Ascomycota, which causes powdery mildew on onion. This disease prefers warm, dry environments. It is rare in the United States, and is currently restricted to western states. Globally, it is also a minor problem with limited occurrences in the Middle East, Europe, and South America. L. taurica causes powdery mildew of onions, but is also known to infect other allium, solanaceous, and cucurbit species. The disease has appeared in parts of the Middle East, the Mediterranean, and South and North America. Currently, it is not a cause for major concern in the U.S. and throughout the world, as its geographic extent is sparse. In addition, it is relatively easy to control through basic sanitation and reducing water stress.
Brasiliomyces malachrae is a species of fungus in the family Erysiphaceae. It is a plant pathogen that grows on Gossypium, Lavatera assurgentiflora, Malachra capitata, Malvastrum coromandelianum, and species of Malvaceae. It is found in South America.

Podosphaera macularis is a plant pathogen infecting several hosts including chamomile, caneberrie, strawberries, hop, hemp and Cineraria. It causes powdery mildew of hops.

Podosphaera pannosa is a plant pathogen. It produces a powdery mildew on members of the rose family.
Erysiphe heraclei is a plant pathogen that causes powdery mildew on several species including dill, carrot and parsley.

Oidium mangiferae is a plant pathogen that infects mango trees causing powdery mildew. Powdery mildew of mango is an Ascomycete pathogen of the Erysiphales family that was initially described by Berthet in 1914, using samples collected from Brazil. O. mangiferae is found in all areas where mangoes have been raised long term, but is particularly widespread in India where both the host and the pathogen are native. Currently no teleomorph stage has been identified, but due to certain morphological characteristics it has been suggested that O. mangiferae belongs in the Erysiphe polygony group. Mango is the only known host for this pathogen, though O. mangiferae appears to be identical to fungi responsible for powdery mildew diseases on various other plant species, particularly oak, though some differences may be observed. In particular, the number of cells in conidiophores varies from 2 on mango to 3-5 on oak. O. mangiferae has been known to infect oak leaves in the laboratory, however due to the lack of a known teleomorph stage O. mangiferae is still considered to only be a pathogen of mango. Recent analysis of its ribosomal DNA suggests it is conspecific with Erysiphe alphitoides, the causative agent of powdery mildew in European oaks.

Podosphaera fuliginea is a plant pathogen that causes powdery mildew on cucurbits. Podosphaera fuliginea and Erysiphe cichoracearum are the two most commonly recorded fungi causing cucurbit powdery mildew. In the past, Erysiphe cichoracearum was considered to be the primary causal organism throughout most of the world. Today, Podosphaera fuliginea is more commonly reported.
Alternaria black spot of canola or grey leaf spot is an ascomycete fungal disease caused by a group of pathogens including: Alternaria brassicae, A. alternata and A. raphani. This pathogen is characterized by dark, sunken lesions of various size on all parts of the plant, including the leaves, stem, and pods. Its primary economic host is canola. In its early stages it only affects the plants slightly by reducing photosynthesis, however as the plant matures it can cause damage to the seeds and more, reducing oil yield as well.

Alternaria brassicicola is a fungal necrotrophic plant pathogen that causes black spot disease on a wide range of hosts, particularly in the genus of Brassica, including a number of economically important crops such as cabbage, Chinese cabbage, cauliflower, oilseeds, broccoli and canola. Although mainly known as a significant plant pathogen, it also contributes to various respiratory allergic conditions such as asthma and rhinoconjunctivitis. Despite the presence of mating genes, no sexual reproductive stage has been reported for this fungus. In terms of geography, it is most likely to be found in tropical and sub-tropical regions, but also in places with high rain and humidity such as Poland. It has also been found in Taiwan and Israel. Its main mode of propagation is vegetative. The resulting conidia reside in the soil, air and water. These spores are extremely resilient and can overwinter on crop debris and overwintering herbaceous plants.

Alternaria leaf spot or Alternaria leaf blight are a group of fungal diseases in plants, that have a variety of hosts. The diseases infects common garden plants, such as cabbage, and are caused by several closely related species of fungi. Some of these fungal species target specific plants, while others have been known to target plant families. One commercially relevant plant genus that can be affected by Alternaria Leaf Spot is Brassica, as the cosmetic issues caused by symptomatic lesions can lead to rejection of crops by distributors and buyers. When certain crops such as cauliflower and broccoli are infected, the heads deteriorate and there is a complete loss of marketability. Secondary soft-rotting organisms can infect stored cabbage that has been affected by Alternaria Leaf Spot by entering through symptomatic lesions. Alternaria Leaf Spot diseases that affect Brassica species are caused by the pathogens Alternaria brassicae and Alternaria brassicicola.

Golovinomyces orontii is a species of fungus that causes powdery mildew disease and it is in the family Erysiphaceae. It is an obligate biotroph that infects plants in several families including Acanthaceae, Asteraceae, Brassicaceae, Cucurbitaceae, and Lamiaceae.
















