
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds such as barbiturates, artificial flavourings, vitamin B1, and vitamin B6.

Cinnamic acid is an organic compound with the formula C6H5-CH=CH-COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a cis and a trans isomer, although the latter is more common.

Ethyl acetate is the organic compound with the formula CH3CO2CH2CH3, simplified to C4H8O2. This colorless liquid has a characteristic sweet smell and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent.

Valeric acid or pentanoic acid is a straight-chain alkyl carboxylic acid with the chemical formula CH3(CH2)3COOH. Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. It is found in the perennial flowering plant Valeriana officinalis, from which it gets its name. Its primary use is in the synthesis of its esters. Salts and esters of valeric acid are known as valerates or pentanoates. Volatile esters of valeric acid tend to have pleasant odors and are used in perfumes and cosmetics. Several, including ethyl valerate and pentyl valerate are used as food additives because of their fruity flavors.
The Simmons–Smith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific.
In organic chemistry, the Knoevenagel condensation reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation.
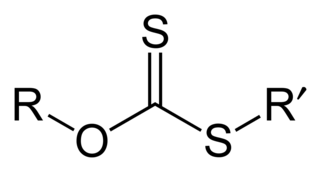
A xanthate is a salt or ester of a xanthic acid. The formula of the salt of xanthic acid is [R−O−CS2]−M+. Xanthate also refers to the anion [R−O−CS2]−. The formula of a xanthic acid is R−O−C(=S)−S−H, such as ethyl xanthic acid, while the formula of an ester of a xanthic acid is R−O−C(=S)−S−R', where R and R' are organyl groups. The salts of xanthates are also called O-organyl dithioates. The esters of xanthic acid are also called O,S-diorganyl esters of dithiocarbonic acid. The name xanthate is derived from Ancient Greek ξανθός (xanthos) meaning 'yellowish' or 'golden', and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and for extraction of certain sulphide bearing ores. They are also versatile intermediates in organic synthesis.
Dodecanol, or lauryl alcohol, is an organic compound produced industrially from palm kernel oil or coconut oil. It is a fatty alcohol. Sulfate esters of lauryl alcohol, especially sodium lauryl sulfate, are very widely used as surfactants. Sodium lauryl sulfate and the related dodecanol derivatives ammonium lauryl sulfate and sodium laureth sulfate are all used in shampoos. Dodecanol is tasteless, colorless, and has a floral odor.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).
Enanthic acid, also called heptanoic acid, is an organic compound composed of a seven-carbon chain terminating in a carboxylic acid functional group. It is a colorless oily liquid with an unpleasant, rancid odor. It contributes to the odor of some rancid oils. It is slightly soluble in water, but very soluble in ethanol and ether. Salts and esters of enanthic acid are called enanthates or heptanoates.

Ethyl benzoate, C9H10O2, is an ester formed by the condensation of benzoic acid and ethanol. It is a colorless liquid that is almost insoluble in water, but miscible with most organic solvents.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 CFR 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.
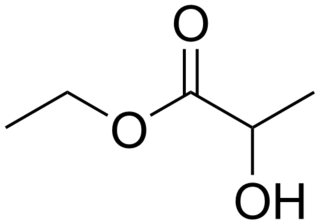
Ethyl lactate, also known as lactic acid ethyl ester, is the organic compound with the formula CH3CH(OH)CO2CH2CH3. It is the ethyl ester of lactic acid. A colorless liquid, it is a chiral ester. Being naturally derived, it is readily available as a single enantiomer. It is commonly used as a solvent. This compound is considered biodegradable and can be used as a water-rinsible degreaser. Ethyl lactate is found naturally in small quantities in a wide variety of foods including wine, chicken, and various fruits. The odor of ethyl lactate when dilute is mild, buttery, creamy, with hints of fruit and coconut.

Ethyl pentanoate, also commonly known as ethyl valerate, is an organic compound used in flavors. It is an ester with the molecular formula C7H14O2. This colorless liquid is poorly soluble in water but miscible with organic solvents.

Ethyl methylphenylglycidate, commonly known as strawberry aldehyde, is an organic compound used in the flavor industry in artificial fruit flavors, in particular strawberry.

Ethyl acrylate is an organic compound with the formula CH2CHCO2CH2CH3. It is the ethyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced for paints, textiles, and non-woven fibers. It is also a reagent in the synthesis of various pharmaceutical intermediates.

Hagemann's ester, ethyl 2-methyl-4-oxo-2-cyclohexenecarboxylate, is an organic compound that was first prepared and described in 1893 by German chemist Carl Hagemann. The compound is used in organic chemistry as a reagent in the synthesis of many natural products including sterols, trisporic acids, and terpenoids.

tert-Butylthiol, also known as tert-butyl mercaptan (TBM), and abbreciated t-BuSH, is an organosulfur compound with the formula (CH3)3CSH. This thiol is used as an odorant for natural gas, which is otherwise odorless. It may also have been used as a flavoring agent.
Decadienoic acid is any mono-carboxylic acid with an unbranched chain of ten carbon atoms, connected by seven single bonds and two double bonds. That is, any compound with formula HO(O=)C–(CH
2)
x–CH=CH–(CH
2)
y–CH=CH–(–CH
2)
z–H where x, y, and z can be zero or more, and x+y+z = 5 ; or HO(O=)C–(CH
2)
r–CH=C=CH–(CH
2)
s–H where r + s = 6. All these compounds have the formula C
10H
16O
2. A salt or ester of such an acid is called a decadienoate.














