In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group (−OH) bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethanol, which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest members, includes all compounds for which the general formula is CnH2n+1OH. Simple monoalcohols that are the subject of this article include primary, secondary and tertiary alcohols.

Methanol, also known as methyl alcohol, amongst other names, is a chemical and the simplest alcohol, with the formula CH3OH (a methyl group linked to a hydroxyl group, often abbreviated MeOH). It is a light, volatile, colourless, flammable liquid with a distinctive alcoholic odour similar to that of ethanol (potable alcohol). A polar solvent, methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide.
Isobutane, also known as i-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH3)3. It is an isomer of butane. Isobutane is a colourless, odourless gas. It is the simplest alkane with a tertiary carbon atom. Isobutane is used as a precursor molecule in the petrochemical industry, for example in the synthesis of isooctane.

Biofuel is a fuel that is produced over a short time span from biomass, rather than by the very slow natural processes involved in the formation of fossil fuels, such as oil. Since biomass can be used as a fuel directly, some people use the words biomass and biofuel interchangeably. However, the word biofuel is usually reserved for liquid or gaseous fuels, used for transportation. The U.S. Energy Information Administration (EIA) follows this naming practice.

Denatured alcohol is ethanol that has additives to make it poisonous, bad-tasting, foul-smelling, or nauseating to discourage its recreational consumption. It is sometimes dyed so that it can be identified visually. Pyridine and methanol, each and together, make denatured alcohol poisonous; and denatonium makes it bitter.

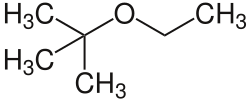
Methyl tertiary-butyl ether (MTBE), also known as methyl tert-butyl ether and tert-butyl methyl ether, is an organic compound with a structural formula (CH3)3COCH3. MTBE is a volatile, flammable, and colorless liquid that is sparingly soluble in water. Primarily used as a fuel additive, MTBE is blended into gasoline to increase knock resistance and reduce unwanted emissions.

Liquid fuels are combustible or energy-generating molecules that can be harnessed to create mechanical energy, usually producing kinetic energy; they also must take the shape of their container. It is the fumes of liquid fuels that are flammable instead of the fluid. Most liquid fuels in widespread use are derived from fossil fuels; however, there are several types, such as hydrogen fuel, ethanol, and biodiesel, which are also categorized as a liquid fuel. Many liquid fuels play a primary role in transportation and the economy.
Methanol fuel is an alternative biofuel for internal combustion and other engines, either in combination with gasoline or independently. Methanol (CH3OH) is less expensive to produce sustainably than ethanol fuel, although it is generally more toxic and has lower energy density. For optimizing engine performance and fuel availability, however, a blend of ethanol, methanol and petroleum is likely to be preferable to using any of these alone. Methanol (a methyl group linked to a hydroxyl group) may be made from hydrocarbon or renewable resources, in particular natural gas and biomass respectively. It can also be synthesized from CO2 (carbon dioxide) and hydrogen. Methanol fuel is currently used by racing cars in many countries but has not seen widespread use otherwise.
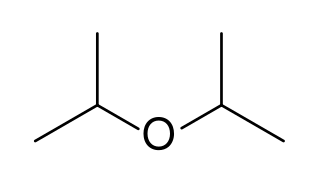
Diisopropyl ether is secondary ether that is used as a solvent. It is a colorless liquid that is slightly soluble in water, but miscible with organic solvents. It is used as an extractant and an oxygenate gasoline additive. It is obtained industrially as a byproduct in the production of isopropanol by hydration of propylene. Diisopropyl ether is sometimes represented by the abbreviation DIPE.

Isobutylene (or 2-methylpropene) is a hydrocarbon with the formula (CH3)2C=CH2. It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.

Methylcyclopentadienyl manganese tricarbonyl (MMT or MCMT) is an organomanganese compound with the formula (C5H4CH3)Mn(CO)3. Initially marketed as a supplement for use in leaded gasoline, MMT was later used in unleaded gasoline to increase the octane rating. Following the implementation of the Clean Air Act (United States) (CAA) in 1970, MMT continued to be used alongside tetraethyl lead (TEL) in the US as leaded gasoline was phased out (prior to TEL finally being banned from US gasoline in 1995), and was also used in unleaded gasoline until 1977. Ethyl Corporation obtained a waiver from the U.S. EPA (Environmental Protection Agency) in 1995, which allows the use of MMT in US unleaded gasoline (not including reformulated gasoline) at a treat rate equivalent to 8.3 mg Mn/L (manganese per liter).

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.
Oxygenated chemical compounds contain oxygen as a part of their chemical structure. The term usually refers to oxygenated chemical compounds added to fuels. Oxygenates are usually employed as gasoline additives to reduce carbon monoxide and soot that is created during the burning of the fuel. Compounds related to soot, such as polyaromatic hydrocarbons (PAHs) and nitrated PAHs, are also reduced.

2,4-Dimethyl-6-tert-butylphenol is the organic compound with the formula Me2(tert-Bu)C6H2OH (Me = methyl, tert-Bu = tertiary butyl). It is a colorless oil that is classified as an alkylated phenol.
An antiknock agent is a gasoline additive used to reduce engine knocking and increase the fuel's octane rating by raising the temperature and pressure at which auto-ignition occurs.
Isopropyl alcohol is a colorless, flammable chemical compound with a strong odor. As an isopropyl group linked to a hydroxyl group, it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of 1-propanol and ethyl methyl ether.
Methyl tert-butyl ether (MTBE) is a gasoline additive that replaced tetraethyllead. MTBE is an oxygenate and raises gasoline's octane number. Its use declined in the United States in response to environmental and health concerns. It has polluted groundwater due to MTBE-containing gasoline being spilled or leaked at gas stations. MTBE spreads more easily underground than other gasoline components due to its higher solubility in water. Cost estimates for removing MTBE from groundwater and contaminated soil range from $1 billion to $30 billion, including removing the compound from aquifers and municipal water supplies, and replacing leaky underground oil tanks. Who will pay for remediation is controversial. In one case, the cost to oil companies to clean up the MTBE in wells belonging to the city of Santa Monica, California is estimated to exceed $200 million.
tert-Amyl methyl ether (TAME) is an ether used as a fuel oxygenate. TAME derives from C5 distillation fractions of naphtha. It has an ethereous odor. Unlike most ethers, it does not require a stabilizer as it does not form peroxides on storage.

tert-Amyl ethyl ether (TAEE) is a chemical compound, classified as an ether, with the molecular formula C7H16O. It is used as an additive in gasoline fuels as an oxygenate and also as a solvent in organic chemistry.