In organometallic chemistry, f-block metallocenes are a class of sandwich compounds consisting of an f-block metal and a set of electron-rich ligands such as the cyclopentadienyl anion.
In organometallic chemistry, f-block metallocenes are a class of sandwich compounds consisting of an f-block metal and a set of electron-rich ligands such as the cyclopentadienyl anion.
The first prepared and well-characterized f-block metallocenes were the tris(cyclopentadienyl) lanthanide complexes, (C5H5)3Ln (Ln = La, Ce, Pr, Nd, Sm and Gd). [1] [2] However, their significance is limited more to their existences and structures than to their reactivity. The cyclopentadienyl ligands of f-block metallocenes were considered as inert ancillary ligands, only capable of enhancing their stability and solubility, but not their reactivity. In addition, only late and small metals in the lanthanide series, i.e., elements from Sm to Lu, are trivalent metallocene complexes, [(C5H5)2LnZ]n [3] [4] In 1980, the pentamethylcyclopentadienyl ligand, C5Me−
5, was introduced to prepare the lanthanide complexes with all metals in the series. [3] [5] [6] [7] [8] Apart from improving the stability and solubility of the complexes, it was demonstrated to participate in organometallic reactions. Subsequently, William J. Evans and his coworkers successfully isolated (C5Me5)2Sm(THF)2 [8] and (C5Me5)2Sm, [9] making a breakthrough in f-block metallocenes, since both of these two organosamarium(II) complexes were unexpectedly found to participate in the coordination, activation and transformation of a variety of unsaturated compounds, including olefins, [8] [10] [11] [12] dinitrogen, [13] internal alkynens, [14] [15] phosphaalkynes, [16] carbon monoxide, [17] carbon dioxide, [18] isonitriles, [19] diazine derivatives, [20] [21] [22] imines [23] and polycyclic aromatic hydrocarbons (PAHs). [24] Moreover, due to its strong reducing potential, it was used to synthesize [(C5Me5)2Sm(μ-H)]2 and other trivalent f-block element complexes. [14] Subsequently, tris(pentamethylcyclopentadienyl) lanthanide complexes, (C5Me5)3Ln, and their relevant complexes were synthesized from Sm2+ complexes. These metallocenes included (C5Me5)3Sm, [(C5H3(SiMe3)2]3Sm, (C5Me5)2Sm(C5H5), [(C5Me5)2Sm]2(μ-C5H5). [25] [26] [27] Later, one tris(pentamethylcyclopentadienyl) f-element halide complex, (C5Me5)3UCl, was successfully isolated as the intermediate of the formation of (C5Me5)2UCl2. [28] It is worthy mentioning that (C5Me5)3UCl has a very similar structure as (C5Me5)3U and its uranium-chloride bond (2.90 Å) is relatively longer than the uranium-chloride bonds of other analogues. [29] Its existence also indicates that the larger f-block elements are capable of accommodating additional ligands in addition to the three cyclopentadienyl ligands resulting in the isolation of the following complexes: (C5Me5)3UF, [28] (C5H3(TMS)2)3Th [30] and (C5Me5)3ThH. [31]
I. the synthesis of the first f-block metallocenes is described by following equation: [1] [2]
II. Preparation of (C5Me5)3Sm:
(i) the first (C5Me5)3Sm was prepared via exploratory Sm2+ chemistry with cyclooctatetraene: [25]
(ii) Similar to method (i), (C5Me5)3Sm can be efficiently synthesized from a Sm2+ precursor and (C5Me5)2Pb: [32]
In this pathway, (C5Me5)2Sm(OEt2) is used since it is more readily available than (C5Me5)3Sm and does not react THF.
(iii) additionally, (C5Me5)3Sm can also be prepared from trivalent precursors, without ring opening THF.
This solvated cation route generally allows the preparation of all (C5Me5)3Ln complexes since [(C5Me5)3LnH]x is known for all lanthanide elements.
An alternative unsolvated cation pathway prohibits THF during the reaction since (C5Me5)3Sm can ring open THF. [33] [34]
III. Generally, in order to synthesize (C5Me5)3M, the starting materials and the reaction conditions require optimizing to ensure (C5Me5)3M is the most favored product. [29] In addition, compounds capable of reacting with (C5Me5)3M, such as THF, nitriles or isonitriles, should be avoided. [29] Therefore, the following routes are possible options:
(i) For M=Ln including La, Ce, Pr, Nd and Gd, unsolvated cation route is preferred since [(C5Me5)2LnH]x complexes are too reactive.
Notably, the synthesis of (C5Me5)3La and (C5Me5)3Ce requires the usage of silylated glassware since they are easily oxidized.
(ii) For M=actinide like U, solvated cation route can be used.
Unlike d-block elements, f-block elements do not follow 18-electron rule due to their f-orbitals. [35] The following complexes, (C5H4SiMe3)3Ln, have extremely negative reduction potentials of -2.7 to -3.9 Volts versus the standard hydrogen electrode (NHE). [36] Furthermore, in comparison with d-orbitals of transition metals, the radial extension of their 4f-orbitals are really small and limited, which greatly reduces the orbital effects. [37] More specifically, its 4fn electron configurations have almost no effect on its chemical reactivity and its electrostatic interactions require optimizing through ligand geometries. Moreover, the reactivity of the f-block element complexes relies heavily on their sterics. In other words, a sterically saturated structure offers the best stability, and so, both ligand size or metal size can be altered to modify the reactivity. [37] These special properties allow the following reactions to occur.
Like alkyl group, the electron-rich ligand of f-block metallocenes can act as a nucleophile during organometallic reactions. For example, they can polymerize olefins, [38] and participate in ring opening polymerizations, [34] [39] etc.
Especially in the presence of Lewis acids like B(C6F5)3 or Al2Me6, the Cp and other similar ligands can be removed in the following way. [40]
The f-block metallocenes are able to undergo insertion reactions of compounds like carbon monoxide, [41] nitriles or isocyanates. [34]
Since f-block metallocenes are very electron-rich, they tend to lose one electron and a pentamethylcyclopentadienyl ligand.
Sterically crowded complexes like (C5Me5)3Sm are able to provide strong reductivity and so this type of reaction was named as SIR. [42] Due to the strong steric hindrance, one ligand cannot bind to the metal center at the ideal distance and so the complex is not stable. [29] Thus, the anion is more inclined to become oxidized and leave the complex, resulting in a highly reducing metal complex.

A metallocene is a compound typically consisting of two cyclopentadienyl anions (C
5H−
5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride or vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization.
Cyclopentadiene is an organic compound with the formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.

Nickelocene is the organonickel compound with the formula Ni(η5-C5H5)2. Also known as bis(cyclopentadienyl)nickel or NiCp2, this bright green paramagnetic solid is of enduring academic interest, although it does not yet have any known practical applications.

Cobaltocene, known also as bis(cyclopentadienyl)cobalt(II) or even "bis Cp cobalt", is an organocobalt compound with the formula Co(C5H5)2. It is a dark purple solid that sublimes readily slightly above room temperature. Cobaltocene was discovered shortly after ferrocene, the first metallocene. Due to the ease with which it reacts with oxygen, the compound must be handled and stored using air-free techniques.

Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.

In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.
Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives. Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.

Group 2 organometallic chemistry refers to the chemistry of compounds containing carbon bonded to any group 2 element. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organometallic group 2 compounds are rare and are typically limited to academic interests.
Ruthenocene is an organoruthenium compound with the formula (C5H5)2Ru. This pale yellow, volatile solid is classified as a sandwich compound and more specifically, as a metallocene.

Decamethyldizincocene is an organozinc compound with the formula [Zn2(η5–C5Me5)2]. It is the first and an unusual example of a compound with a Zn-Zn bond. Decamethyldizincocene is a colorless crystalline solid that burns spontaneously in the presence of oxygen and reacts with water. It is stable at room temperature and especially soluble in diethyl ether, benzene, pentane, or tetrahydrofuran.

Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.
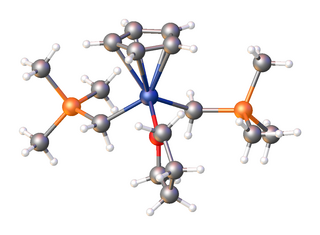
Organoscandium chemistry is an area with organometallic compounds focused on compounds with at least one carbon to scandium chemical bond. The interest in organoscandium compounds is mostly academic but motivated by potential practical applications in catalysis, especially in polymerization. A common precursor is scandium chloride, especially its THF complex.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.
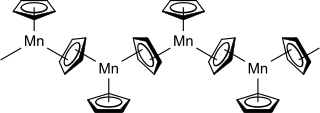
Manganocene or bis(cyclopentadienyl)manganese(II) is an organomanganese compound with the formula [Mn(C5H5)2]n. It is a thermochromic solid that degrades rapidly in air. Although the compound is of little utility, it is often discussed as an example of a metallocene with ionic character.
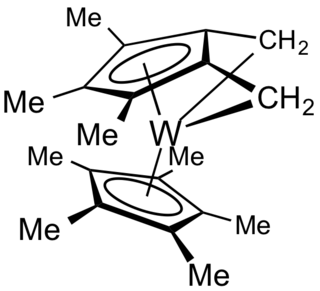
In organometallic chemistry, a tuck-in complex usually refers to derivatives of Cp* ligands wherein a methyl group is deprotonated and the resulting methylene attaches to the metal. The C5–CH2–M angle is acute. The term "tucked in" was coined to describe derivatives of organotungsten complexes. Although most "tucked-in" complexes are derived from Cp* ligands, other pi-bonded rings undergo similar reactions.
William J. Evans is a Distinguished Professor at the University of California, Irvine, who specializes in the inorganic and organometallic chemistry of heavy metals, specifically the rare earth metals, actinides, and bismuth. He has published over 500 peer-reviewed research papers on these topics.
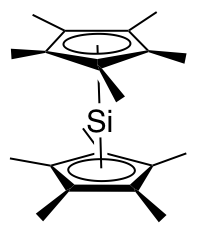
Decamethylsilicocene, (C5Me5)2Si, is a group 14 sandwich compound. It is an example of a main-group cyclopentadienyl complex; these molecules are related to metallocenes but contain p-block elements as the central atom. It is a colorless, air sensitive solid that sublimes under vacuum.

A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand.

Stannocene is an organometallic compound with the formula Sn(C5H5)2. It is a metallocene that can be produced efficiently from cyclopentadienyl sodium and tin(II) chloride. Unlike in ferrocene the two cyclopentadienyl rings are not parallel.