
Chalcopyrite ( KAL-kə-PY-ryte, -koh-) is a copper iron sulfide mineral and the most abundant copper ore mineral. It has the chemical formula CuFeS2 and crystallizes in the tetragonal system. It has a brassy to golden yellow color and a hardness of 3.5 to 4 on the Mohs scale. Its streak is diagnostic as green tinged black.

Sphalerite is a sulfide mineral with the chemical formula (Zn,Fe)S. It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in sedimentary exhalative, Mississippi-Valley type, and volcanogenic massive sulfide deposits. It is found in association with galena, chalcopyrite, pyrite, calcite, dolomite, quartz, rhodochrosite, and fluorite.

Bornite, also known as peacock ore, is a sulfide mineral with chemical composition Cu5FeS4 that crystallizes in the orthorhombic system (pseudo-cubic).

Tennantite is a copper arsenic sulfosalt mineral with an ideal formula Cu12As4S13. Due to variable substitution of the copper by iron and zinc the formula is Cu6[Cu4(Fe,Zn)2]As4S13. It is gray-black, steel-gray, iron-gray or black in color. A closely related mineral, tetrahedrite (Cu12Sb4S13) has antimony substituting for arsenic and the two form a solid solution series. The two have very similar properties and is often difficult to distinguish between tennantite and tetrahedrite. Iron, zinc, and silver substitute up to about 15% for the copper site.

In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

A single-displacement reaction, also known as single replacement reaction or exchange reaction, is a chemical reaction in which one element is replaced by another in a compound.

Tetrahedrite is a copper antimony sulfosalt mineral with formula: (Cu,Fe)
12Sb
4S
13. It is the antimony endmember of the continuous solid solution series with arsenic-bearing tennantite. Pure endmembers of the series are seldom if ever seen in nature. Of the two, the antimony rich phase is more common. Other elements also substitute in the structure, most notably iron and zinc, along with less common silver, mercury and lead. Bismuth also substitutes for the antimony site and bismuthian tetrahedrite or annivite is a recognized variety. The related, silver dominant, mineral species freibergite, although rare, is notable in that it can contain up to 18% silver.

The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide (S22−) as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenides, the tellurides, the arsenides, the antimonides, the bismuthinides, the sulfarsenides and the sulfosalts. Sulfide minerals are inorganic compounds.

Enargite is a copper arsenic sulfosalt mineral with formula Cu3AsS4. It takes its name from the Greek word enarge, "distinct". Enargite is a steel gray, blackish gray, to violet black mineral with metallic luster. It forms slender orthorhombic prisms as well as massive aggregates. It has a hardness of 3 and a specific gravity of 4.45.

Sulfosalt minerals are those complex sulfide minerals with the general formula: AmBnSp; where A represents a metal such as copper, lead, silver, iron, and rarely mercury, zinc, vanadium; B usually represents semi-metal such as arsenic, antimony, bismuth, and rarely germanium, or metals like tin and rarely vanadium; and S is sulfur or rarely selenium or/and tellurium. The Strunz classification includes the sulfosalts in a sulfides and sulfosalts superclass. A group which have similar appearing formulas are the sulfarsenides. In sulfarsenides the arsenic substitutes for sulfide anions whereas in the sulfosalts the arsenic substitutes for a metal cation.
In ore deposit geology, supergene processes or enrichment are those that occur relatively near the surface as opposed to deep hypogene processes. Supergene processes include the predominance of meteoric water circulation (i.e. water derived from precipitation) with concomitant oxidation and chemical weathering. The descending meteoric waters oxidize the primary (hypogene) sulfide ore minerals and redistribute the metallic ore elements. Supergene enrichment occurs at the base of the oxidized portion of an ore deposit. Metals that have been leached from the oxidized ore are carried downward by percolating groundwater, and react with hypogene sulfides at the supergene-hypogene boundary. The reaction produces secondary sulfides with metal contents higher than those of the primary ore. This is particularly noted in copper ore deposits where the copper sulfide minerals chalcocite (Cu2S), covellite (CuS), digenite (Cu18S10), and djurleite (Cu31S16) are deposited by the descending surface waters.

In chemistry isomorphism has meanings both at the level of crystallography and at a molecular level. In crystallography, compounds are isomorphous if their symmetry is the same and their unit cell parameters are similar
Cleusonite is a member of the crichtonite group of minerals with the chemical formula (Pb,Sr)(U4+
,U6+
)(Fe2+
,Zn)
2(Ti,Fe2+
,Fe3+
)
18(O,OH)
38. This group of minerals contains approximately thirteen complex metal titanates. The structures of minerals of this group is complicated by frequent fine-scale twinning and metamictization due to radioactive elements. The crichtonite group consists of members of related mineral species of the type A{BC2D6E12}O38 which are characterized by their predominant cations (as seen in crichtonite (Sr), senaite (Pb), davidite (REE + U), landauite (Na), loveringite (Ca), lindsleyite (Ba), and mathiasite (K).
This list gives an overview of the classification of non-silicate minerals and includes mostly International Mineralogical Association (IMA) recognized minerals and its groupings. This list complements the List of minerals recognized by the International Mineralogical Association series of articles and List of minerals. Rocks, ores, mineral mixtures, not IMA approved minerals, not named minerals are mostly excluded. Mostly major groups only, or groupings used by New Dana Classification and Mindat.

Jalpaite is a rare copper silver sulfide mineral with formula Ag3CuS2.

Kësterite is a sulfide mineral with a chemical formula of Cu2(Zn,Fe)SnS4. In its lattice structure, zinc and iron atoms share the same lattice sites. Kesterite is the Zn-rich variety whereas the Zn-poor form is called ferrokesterite or stannite. Owing to their similarity, kesterite is sometimes called isostannite. The synthetic form of kesterite is abbreviated as CZTS. The name kesterite is sometimes extended to include this synthetic material and also CZTSe, which contains selenium instead of sulfur.
Oxyphosphides are chemical compounds formally containing the group PO, with one phosphorus and one oxygen atom. The phosphorus and oxygen are not bound together as in phosphates or phosphine oxides, instead they are bound separately to the cations (metals), and could be considered as a mixed phosphide-oxide compound. So a compound with OmPn requires cations to balance a negative charge of 2m+3n. The cations will have charges of +2 or +3. The trications are often rare earth elements or actinides. They are in the category of oxy-pnictide compounds.
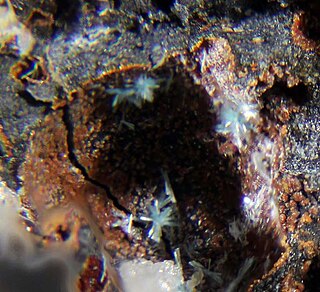
Mammothite is a mineral found in the Mammoth mine in Tiger, Arizona and also in Laurium, Attika, Greece. This mineral was named in 1985 by Donald R. Peacor, Pete J. Dunn, G. Schnorrer-Köhler, and Richard A. Bideaux, for the Mammoth vein (one of the two main veins in the mine) and the town of Mammoth, Arizona, which was named for the mine. The mammothite that is found in Arizona exist as euhedral crystals imbedded in micro granular, white colored anglesite with a saccharoidal texture. The associated minerals include phosgenite, wulfenite, leadhillite and caledonite. In Greece, the mammothite exists as small euhedral crystals and also as microscopic rock cavities lined with projecting crystals within the slags. The associated minerals here are cerussite, phosgenite and matlockite. The ideal chemical formula for mammothite is Pb6Cu4AlSb5+O2(OH)16Cl4(SO4)2.














