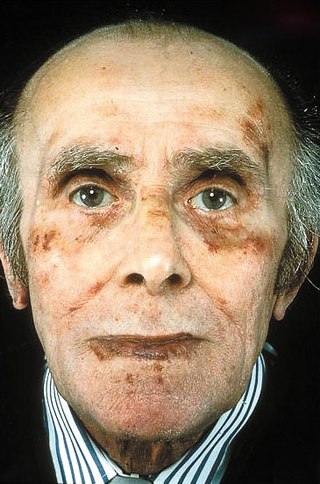
Amyloidosis is a group of diseases in which abnormal proteins, known as amyloid fibrils, build up in tissue. There are several non-specific and vague signs and symptoms associated with amyloidosis. These include fatigue, peripheral edema, weight loss, shortness of breath, palpitations, and feeling faint with standing. In AL amyloidosis, specific indicators can include enlargement of the tongue and periorbital purpura. In wild-type ATTR amyloidosis, non-cardiac symptoms include: bilateral carpal tunnel syndrome, lumbar spinal stenosis, biceps tendon rupture, small fiber neuropathy, and autonomic dysfunction.

Transthyretin (TTR or TBPA) is a transport protein in the plasma and cerebrospinal fluid that transports the thyroid hormone thyroxine (T4) and retinol to the liver. This is how transthyretin gained its name: transports thyroxine and retinol. The liver secretes TTR into the blood, and the choroid plexus secretes TTR into the cerebrospinal fluid.

Cerebral amyloid angiopathy (CAA) is a form of angiopathy in which amyloid beta peptide deposits in the walls of small to medium blood vessels of the central nervous system and meninges. The term congophilic is sometimes used because the presence of the abnormal aggregations of amyloid can be demonstrated by microscopic examination of brain tissue after staining with Congo red. The amyloid material is only found in the brain and as such the disease is not related to other forms of amyloidosis.
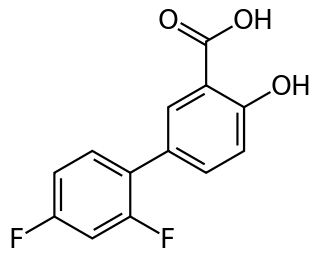
Diflunisal is a salicylic acid derivative with analgesic and anti-inflammatory activity. It was developed by Merck Sharp & Dohme in 1971, as MK647, after showing promise in a research project studying more potent chemical analogs of aspirin. It was first sold under the brand name Dolobid, marketed by Merck & Co., but generic versions are now widely available. It is classed as a nonsteroidal anti-inflammatory drug (NSAID) and is available in 250 mg and 500 mg tablets.

Familial amyloid polyneuropathy, also called transthyretin-related hereditary amyloidosis, transthyretin amyloidosis abbreviated also as ATTR, or Corino de Andrade's disease, is an autosomal dominant neurodegenerative disease. It is a form of amyloidosis, and was first identified and described by Portuguese neurologist Mário Corino da Costa Andrade, in 1952. FAP is distinct from senile systemic amyloidosis (SSA), which is not inherited, and which was determined to be the primary cause of death for 70% of supercentenarians who have been autopsied. FAP can be ameliorated by liver transplantation.
Small fiber peripheral neuropathy is a type of peripheral neuropathy that occurs from damage to the small unmyelinated and myelinated peripheral nerve fibers. These fibers, categorized as C fibers and small Aδ fibers, are present in skin, peripheral nerves, and organs. The role of these nerves is to innervate some skin sensations and help control autonomic function. It is estimated that 15–20 million people in the United States have some form of peripheral neuropathy.

Cardiac amyloidosis is a subcategory of amyloidosis where there is depositing of the protein amyloid in the cardiac muscle and surrounding tissues. Amyloid, a misfolded and insoluble protein, can become a deposit in the heart's atria, valves, or ventricles. These deposits can cause thickening of different sections of the heart, leading to decreased cardiac function. The overall decrease in cardiac function leads to a plethora of symptoms. This multisystem disease was often misdiagnosed, with a corrected analysis only during autopsy. Advancements of technologies have increased earlier accuracy of diagnosis. Cardiac amyloidosis has multiple sub-types including light chain, familial, and senile. One of the most studied types is light chain cardiac amyloidosis. Prognosis depends on the extent of the deposits in the body and the type of amyloidosis. New treatment methods are actively being researched in regards to the treatment of heart failure and specific cardiac amyloidosis problems.
Hereditary sensory and autonomic neuropathy (HSAN) or hereditary sensory neuropathy (HSN) is a condition used to describe any of the types of this disease which inhibit sensation.

Familial renal amyloidosis is a form of amyloidosis primarily presenting in the kidney.
Ardalan–Shoja–Kiuru syndrome is a clinical syndrome featuring hereditary gelsolin amyloidosis and retinitis pigmentosa. This syndrome was first recognized by two Iranian physicians, Mohammad Ardalan and Mohammadali Shoja and Finnish neurologist Sari Kiuru-Enari in an Iranian family. Hereditary gelsolin amyloidosis has originally been reported by Finnish ophthalmologist Jouko Meretoja and is known as Meretoja syndrome or Familial Amyloidosis, Finnish type. In addition to the classic manifestations of Finnish type Familial Amyloidosis, cutis laxa, progressive peripheral neuropathy and corneal lattice dystrophy, some of the affected members of the Iranian family have retinitis pigmentosa. This feature had not been previously reported with this type of amyloidosis. Ardalan–Shoja–Kiuru syndrome or hereditary gelsolin amyloidosis plus retinitis pigmentosa has not been found outside this single Iranian family.

Tafamidis, sold under the brand names Vyndaqel and Vyndamax, is a medication used to delay disease progression in adults with certain forms of transthyretin amyloidosis. It can be used to treat both hereditary forms, familial amyloid cardiomyopathy and familial amyloid polyneuropathy, as well as wild-type transthyretin amyloidosis, which formerly was called senile systemic amyloidosis. It works by stabilizing the quaternary structure of the protein transthyretin. In people with transthyretin amyloidosis, transthyretin falls apart and forms clumps called (amyloid) that harm tissues including nerves and the heart.

Lattice corneal dystrophy type is a rare form of corneal dystrophy. It has no systemic manifestations, unlike the other type of the dystrophy, Lattice corneal dystrophy type II. Lattice corneal dystrophy was first described by Swiss ophthalmologist Hugo Biber in 1890.

Familial Amyloidosis, Finnish Type (FAF), also called hereditary gelsolin amyloidosis and AGel amyloidosis (AGel), is an amyloid condition with a number of associated cutaneous and neurological presentations deriving from the aberrant proteolysis of a mutated form of plasma gelsolin. First described in 1969 by the Finnish ophthalmologist Jouko Meretoja, FAF is uncommon with 400–600 cases described in Finland and 15 elsewhere.
Familial amyloid cardiomyopathy (FAC), or transthyretin amyloid cardiomyopathy (ATTR-CM) results from the aggregation and deposition of mutant and wild-type transthyretin (TTR) protein in the heart. TTR is usually circulated as a homo-tetramer—a protein made up of four identical subunits—however, in FAC populations, TTR dissociates from this typical form and misassembles into amyloid fibrils which are insoluble and resistant to degradation. Due to this resistance to degradation, when amyloid fibrils accumulate in the heart's walls, specifically the left ventricle, rigidity prevents the heart from properly relaxing and refilling with blood: this is called diastolic dysfunction which can ultimately lead to heart failure.
Alnylam Pharmaceuticals, Inc. is an American biopharmaceutical company focused on the discovery, development and commercialization of RNA interference (RNAi) therapeutics for genetically defined diseases. The company was founded in 2002 and is headquartered in Cambridge, Massachusetts. In 2016, Forbes included the company on its "100 Most Innovative Growth Companies" list.
Chemical chaperones are a class of small molecules that function to enhance the folding and/or stability of proteins. Chemical chaperones are a broad and diverse group of molecules, and they can influence protein stability and polypeptide organization through a variety of mechanisms. Chemical chaperones are used for a range of applications, from production of recombinant proteins to treatment of protein misfolding in vivo.
Wild-type transthyretin amyloid (WTTA), also known as senile systemic amyloidosis (SSA), is a disease that typically affects the heart and tendons of elderly people. It is caused by the accumulation of a wild-type protein called transthyretin. This is in contrast to a related condition called transthyretin-related hereditary amyloidosis where a genetically mutated transthyretin protein tends to deposit much earlier than in WTTA due to abnormal conformation and bioprocessing. It belongs to a group of diseases called amyloidosis, chronic progressive conditions linked to abnormal deposition of normal or abnormal proteins, because these proteins are misshapen and cannot be properly degraded and eliminated by the cell metabolism.
Michael James Polydefkis is an American neurologist. He is a Professor of Neurology at Johns Hopkins University School of Medicine and Co-Director of the Cutaneous Nerve Laboratory. Polydefkis research focuses on treating hATTR amyloidosis and diabetic and HIV-associated peripheral neuropathy.
Electrochemical skin conductance (ESC) is an objective, non-invasive and quantitative electrophysiological measure. It is based on reverse iontophoresis and (multiple) steady chronoamperometry.
Vutrisiran, previously known as (ALN-TTRSC02), sold under the brand name Amvuttra, is a medication used for the treatment of the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. It is a double stranded small interfering RNA (siRNA) that interferes with the expression of the transthyretin (TTR) gene. Transthyretin is a serum protein made in the liver whose major function is transport of vitamin A and thyroxine. Rare mutations in the transthyretin gene result in accumulation of large amyloid deposits of misfolded transthyretin molecules most prominently in peripheral nerves and the heart. Patients with hATTR typically present with polyneuropathy or autonomic dysfunction followed by cardiomyopathy which, if untreated, is fatal within 5 to 10 years.







