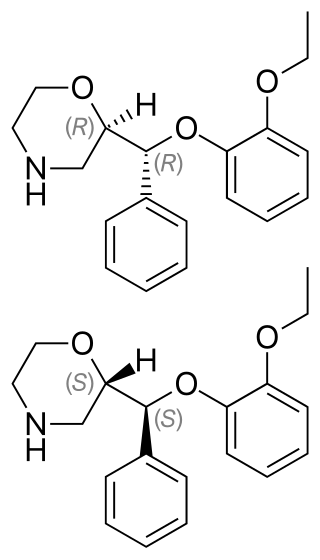
Reboxetine, sold under the brand name Edronax among others, is a selective norepinephrine reuptake inhibitor (sNRI) medication marketed as an antidepressant by Pfizer for use in the treatment of major depressive disorder, although it has also been used off-label for panic disorder and attention deficit hyperactivity disorder (ADHD). It is approved for use in many countries worldwide, but has not been approved for use in the United States.

Idarubicin or 4-demethoxydaunorubicin is an anthracycline antileukemic drug. It inserts itself into DNA and prevents DNA unwinding by interfering with the enzyme topoisomerase II. It is an analog of daunorubicin, but the absence of a methoxy group increases its fat solubility and cellular uptake. Similar to other anthracyclines, it also induces histone eviction from chromatin.

Cabergoline, sold under the brand name Dostinex among others, is a dopaminergic medication used in the treatment of high prolactin levels, prolactinomas, Parkinson's disease, and for other indications. It is taken by mouth.

Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer. This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia. It is often used together with other chemotherapy agents. Doxorubicin is given by injection into a vein.

Daunorubicin, also known as daunomycin, is a chemotherapy medication used to treat cancer. Specifically it is used for acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myelogenous leukemia (CML), and Kaposi's sarcoma. It is administered by injection into a vein. A liposomal formulation known as liposomal daunorubicin also exists.

Anthracyclines are a class of drugs used in cancer chemotherapy that are extracted from Streptomyces peucetius bacterium. These compounds are used to treat many cancers, including leukemias, lymphomas, breast, stomach, uterine, ovarian, bladder cancer, and lung cancers. The first anthracycline discovered was daunorubicin, which is produced naturally by Streptomyces peucetius, a species of Actinomycetota. Clinically the most important anthracyclines are doxorubicin, daunorubicin, epirubicin and idarubicin.

Epirubicin is an anthracycline drug used for chemotherapy. It can be used in combination with other medications to treat breast cancer in patients who have had surgery to remove the tumor. It is marketed by Pfizer under the trade name Ellence in the US and Pharmorubicin or Epirubicin Ebewe elsewhere.
Extravasation is the leakage of intravenously (IV) infused, and potentially damaging, medications into the extravascular tissue around the site of infusion. The leakage can occur through brittle veins in the elderly, through previous venipuncture access, or through direct leakage from wrongly positioned venous access devices. When the leakage is not of harmful consequence it is known as infiltration. Extravasation of medication during intravenous therapy is an adverse event related to therapy that, depending on the medication, amount of exposure, and location, can potentially cause serious injury and permanent harm, such as tissue necrosis. Milder consequences of extravasation include irritation, characterized by symptoms of pain and inflammation, with the clinical signs of warmth, erythema (redness), or tenderness.

Rifabutin (Rfb) is an antibiotic used to treat tuberculosis and prevent and treat Mycobacterium avium complex. It is typically only used in those who cannot tolerate rifampin such as people with HIV/AIDS on antiretrovirals. For active tuberculosis it is used with other antimycobacterial medications. For latent tuberculosis it may be used by itself when the exposure was with drug-resistant TB.

Dexrazoxane hydrochloride, sold under the brand name Zinecard among others, is a cardioprotective agent. It was discovered in 1972. The IV administration of dexrazoxane is in acidic condition with HCl adjusting the pH.

Breast cancer chemotherapy refers to the use of cytotoxic drugs (chemotherapy) in the treatment of breast cancer.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of Streptomyces. In contrast, only one known non-wild type species, Streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.

Indalpine, sold under the brand name Upstène, is a selective serotonin reuptake inhibitor (SSRI) that was briefly marketed as an antidepressant for treatment of depression. It was marketed in France and a few other European countries.
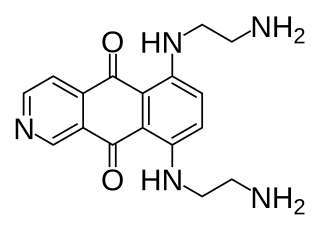
Pixantrone is an experimental antineoplastic (anti-cancer) drug, an analogue of mitoxantrone with fewer toxic effects on cardiac tissue. It acts as a topoisomerase II poison and intercalating agent. The code name BBR 2778 refers to pixantrone dimaleate, the actual substance commonly used in clinical trials.

Aclarubicin (INN) or aclacinomycin A is an anthracycline drug that is used in the treatment of cancer in China. It was previously approved for use in Europe but was discontinued in 2004 due to being rarely prescribed and unprofitable.

Safinamide, sold under the brand name Xadago, is a medication used as treatment for Parkinson's disease with "off" episodes; it has multiple modes of action, including the inhibition of monoamine oxidase B.
Streptomyces peucetius is a bacterium species in the genus Streptomyces.

Daunorubicin/cytarabine, sold under the brand name Vyxeos, is a fixed-dose combination medication used for the treatment of acute myeloid leukemia. It contains the liposomal bound daunorubicin, an anthracycline topoisomerase inhibitor, and cytarabine, a nucleoside metabolic inhibitor.
Selective norepinephrine reuptake inhibitors (sNRIs) are a class of drugs that have been marketed as antidepressants and are used for various mental disorders, mainly depression and attention deficit hyperactivity disorder (ADHD). The norepinephrine transporter (NET) serves as the fundamental mechanism for the inactivation of noradrenergic signaling because of the NET termination in the reuptake of norepinephrine (NE). The selectivity and mechanism of action for the NRI drugs remain mostly unresolved and, to date, only a limited number of NRI-selective inhibitors are available. The first commercially available selective NRI was the drug reboxetine (Edronax), developed as a first-line therapy for major depressive disorder. Atomoxetine (Strattera) is another potent and selective NRI which is also effective and well tolerated for the treatment of ADHD in adults, particularly for those patients at risk of substance abuse.














