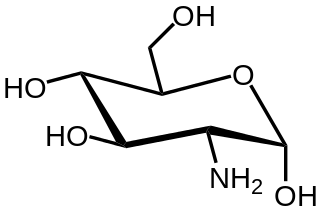
In organic chemistry, an amino sugar is a sugar molecule in which a hydroxyl group has been replaced with an amine group. More than 60 amino sugars are known, with one of the most abundant being N-Acetyl-d-glucosamine, which is the main component of chitin.
The Perkin reaction is an organic reaction developed by English chemist William Henry Perkin that is used to make cinnamic acids. It gives an α,β-unsaturated aromatic acid or α-substituted β-aryl acrylic acid by the aldol condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid. The alkali salt acts as a base catalyst, and other bases can be used instead.
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most often, the Wittig reaction is used to introduce a methylene group using methylenetriphenylphosphorane (Ph3P=CH2). Using this reagent, even a sterically hindered ketone such as camphor can be converted to its methylene derivative.

The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl.
Glycal is a name for cyclic enol ether derivatives of sugars having a double bond between carbon atoms 1 and 2 of the ring. The term “glycal” should not be used for an unsaturated sugar that has a double bond in any position other than between carbon atoms 1 and 2.
In organic chemistry, the Arndt–Eistert reaction is the conversion of a carboxylic acid to its homologue. Named for the German chemists Fritz Arndt (1885–1969) and Bernd Eistert (1902–1978), the method entails treating an acid chlorides with diazomethane. It is a popular method of producing β-amino acids from α-amino acids.

The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid but most of the time either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.

The Peterson olefination is the chemical reaction of α-silyl carbanions with ketones to form a β-hydroxysilane (2) which eliminates to form alkenes (3).

The Ramberg–Bäcklund reaction is an organic reaction converting an α-halo sulfone into an alkene in presence of a base with extrusion of sulfur dioxide. The reaction is named after the two Swedish chemists Ludwig Ramberg and Birger Bäcklund. The carbanion formed by deprotonation gives an unstable episulfone that decomposes with elimination of sulfur dioxide. This elimination step is considered to be a concerted cycloelimination.

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.
The Meyer–Schuster rearrangement is the chemical reaction described as an acid-catalyzed rearrangement of secondary and tertiary propargyl alcohols to α,β-unsaturated ketones if the alkyne group is internal and α,β-unsaturated aldehydes if the alkyne group is terminal. Reviews have been published by Swaminathan and Narayan, Vartanyan and Banbanyan, and Engel and Dudley, the last of which describes ways to promote the Meyer–Schuster rearrangement over other reactions available to propargyl alcohols.
The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, converting a vinyl-substituted cyclopropane ring into a cyclopentene ring.
A chemical glycosylation reaction involves the coupling of a glycosyl donor, to a glycosyl acceptor forming a glycoside. If both the donor and acceptor are sugars, then the product is an oligosaccharide. The reaction requires activation with a suitable activating reagent. The reactions often result in a mixture of products due to the creation of a new stereogenic centre at the anomeric position of the glycosyl donor. The formation of a glycosidic linkage allows for the synthesis of complex polysaccharides which may play important roles in biological processes and pathogenesis and therefore having synthetic analogs of these molecules allows for further studies with respect to their biological importance.
The Ferrier carbocyclization is an organic reaction that was first reported by the carbohydrate chemist Robert J. Ferrier in 1979. It is a metal-mediated rearrangement of enol ether pyrans to cyclohexanones. Typically, this reaction is catalyzed by mercury salts, specifically mercury(II) chloride.
The Tipson–Cohen reaction is a name reaction first discovered by Stuart Tipson and Alex Cohen at the National Bureau of Standards in Washington D.C. The Tipson–Cohen reaction occurs when two neighboring secondary sulfonyloxy groups in a sugar molecule are treated with zinc dust (Zn) and sodium iodide (NaI) in a refluxing solvent such as N,N-dimethylformamide (DMF) to give an unsaturated carbohydrate.

Organoindium chemistry is the chemistry of compounds containing In-C bonds. The main application of organoindium chemistry is in the preparation of semiconducting components for microelectronic applications. The area is also of some interest in organic synthesis. Most organoindium compounds feature the In(III) oxidation state, akin to its lighter congeners Ga(III) and B(III).
The [2,3]-Wittig rearrangement is the transformation of an allylic ether into a homoallylic alcohol via a concerted, pericyclic process. Because the reaction is concerted, it exhibits a high degree of stereocontrol, and can be employed early in a synthetic route to establish stereochemistry. The Wittig rearrangement requires strongly basic conditions, however, as a carbanion intermediate is essential. [1,2]-Wittig rearrangement is a competitive process.
An insertion reaction is a chemical reaction where one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:
Fétizon oxidation is the oxidation of primary and secondary alcohols utilizing the compound silver(I) carbonate absorbed onto the surface of celite also known as Fétizon's reagent first employed by Marcel Fétizon in 1968. It is a mild reagent, suitable for both acid and base sensitive compounds. Its great reactivity with lactols makes the Fétizon oxidation a useful method to obtain lactones from a diol. The reaction is inhibited significantly by polar groups within the reaction system as well as steric hindrance of the α-hydrogen of the alcohol.

Robert John "Robin" Ferrier FRSNZ, FNZIC, was an organic chemist who discovered two chemical reactions, the Ferrier rearrangement and the Ferrier carbocyclization. Originally from Edinburgh, he moved to Wellington, New Zealand, in 1970.










