
Stainless steel, also known as inox, corrosion-resistant steel (CRES) and rustless steel, is an alloy of iron that is resistant to rusting and corrosion. It contains at least 10.5% chromium and usually nickel, as well as 0.2 to 2.11% carbon. Stainless steel's resistance to corrosion results from the chromium, which forms a passive film that can protect the material and self-heal in the presence of oxygen.

Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902). It exists at room temperature in some stainless steels due to the presence of nickel stabilizing the austenite at lower temperatures.
Surgical stainless steel is a grade of stainless steel used in biomedical applications. The most common "surgical steels" are austenitic SAE 316 stainless and martensitic SAE 440, SAE 420, and 17-4 stainless steels. There is no formal definition on what constitutes a "surgical stainless steel", so product manufacturers and distributors often apply the term to refer to any grade of corrosion resistant steel.

Martensitic stainless steel is a type of stainless steel alloy that has a martensite crystal structure. It can be hardened and tempered through aging and heat treatment. The other main types of stainless steel are austenitic, ferritic, duplex, and precipitation hardened.

Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
Cryogenic hardening is a cryogenic treatment process where the material is cooled to approximately −185 °C (−301 °F), usually using liquid nitrogen. It can have a profound effect on the mechanical properties of certain steels, provided their composition and prior heat treatment are such that they retain some austenite at room temperature. It is designed to increase the amount of martensite in the steel's crystal structure, increasing its strength and hardness, sometimes at the cost of toughness. Presently this treatment is being used on tool steels, high-carbon, high-chromium steels and in some cases to cemented carbide to obtain excellent wear resistance. Recent research shows that there is precipitation of fine carbides in the matrix during this treatment which imparts very high wear resistance to the steels.

A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Key characteristics of a superalloy include mechanical strength, thermal creep deformation resistance, surface stability, and corrosion and oxidation resistance.
The weldability, also known as joinability, of a material refers to its ability to be welded. Many metals and thermoplastics can be welded, but some are easier to weld than others. A material's weldability is used to determine the welding process and to compare the final weld quality to other materials.
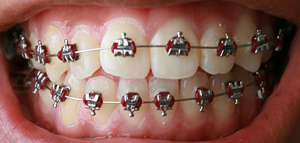
An archwire in orthodontics is a wire conforming to the alveolar or dental arch that can be used with dental braces as a source of force in correcting irregularities in the position of the teeth. An archwire can also be used to maintain existing dental positions; in this case it has a retentive purpose.

Chromium(II) carbide is a ceramic compound that exists in several chemical compositions: Cr3C2, Cr7C3, and Cr23C6. At standard conditions it exists as a gray solid. It is extremely hard and corrosion resistant. It is also a refractory compound, which means that it retains its strength at high temperatures as well. These properties make it useful as an additive to metal alloys. When chromium carbide crystals are integrated into the surface of a metal it improves the wear resistance and corrosion resistance of the metal, and maintains these properties at elevated temperatures. The hardest and most commonly used composition for this purpose is Cr3C2.

In materials science, intergranular corrosion (IGC), also known as intergranular attack (IGA), is a form of corrosion where the boundaries of crystallites of the material are more susceptible to corrosion than their insides.
Austenitic stainless steel is one of the five classes of stainless steel by crystalline structure. Its primary crystalline structure is austenite and it prevents steels from being hardenable by heat treatment and makes them essentially non-magnetic. This structure is achieved by adding enough austenite-stabilizing elements such as nickel, manganese and nitrogen. The Incoloy family of alloys belong to the category of super austenitic stainless steels.

The SAE steel grades system is a standard alloy numbering system for steel grades maintained by SAE International.
Alloy steel is steel that is alloyed with a variety of elements in total amounts between 1.0% and 50% by weight to improve its mechanical properties.
Zeron 100 is a super duplex stainless steel developed by Rolled Alloys. The alloy has excellent corrosion resistance combined with high strength. It typically contains 25% chromium and 7% nickel and 3.6% molybdenum along with copper and tungsten additions. Zeron 100 has a 50–50 austenitic–ferritic structure. It also has greater resistance to chloride pitting, crevice corrosion and stress corrosion cracking than exhibited by the standard 300 series stainless steels.
Duplex stainless steels are a family of stainless steels. These are called duplex grades because their metallurgical structure consists of two phases, austenite and ferrite in roughly equal proportions. They are designed to provide better corrosion resistance, particularly chloride stress corrosion and chloride pitting corrosion, and higher strength than standard austenitic stainless steels such as type A2/304 or A4/316. The main differences in composition, when compared with an austenitic stainless steel is that the duplex steels have a higher chromium content, 20–28%; higher molybdenum, up to 5%; lower nickel, up to 9% and 0.05–0.50% nitrogen. Both the low nickel content and the high strength give significant cost benefits. They are therefore used extensively in the offshore oil and gas industry for pipework systems, manifolds, risers, etc. and in the petrochemical industry in the form of pipelines and pressure vessels. In addition to the improved corrosion resistance compared with the 300 series duplex stainless steels also have higher strength. For example, a Type 304 stainless steel has a 0.2% proof strength in the region of 280 MPa (41 ksi), a 22%Cr duplex stainless steel a minimum 0.2% proof strength of some 450 MPa (65 ksi) and a superduplex grade a minimum of 550 MPa (80 ksi).

SAE 304 stainless steel is the most common stainless steel. It is an alloy of iron, carbon, chromium and nickel. It is an austenitic stainless steel, and is therefore not magnetic. It is less electrically and thermally conductive than carbon steel. It has a higher corrosion resistance than regular steel and is widely used because of the ease in which it is formed into various shapes.
Havar, or UNS R30004, is an alloy of cobalt, possessing a very high mechanical strength. It can be heat-treated. It is highly resistant to corrosion and is non-magnetic. It is biocompatible. It has high fatigue resistance. It is a precipitation hardening superalloy.

Duplex stainless steels are a family of alloys with a two-phase microstructure consisting of both austenitic and ferritic phases. They offer excellent mechanical properties, corrosion resistance, and toughness compared to other types of stainless steel. However, duplex stainless steel can be susceptible to a phenomenon known as 475 °C embrittlement or duplex stainless steel age hardening, which is a type of aging process that causes loss of plasticity in duplex stainless steel when it is heated in the range of 250 to 550 °C. At this temperature range, spontaneous phase separation of the ferrite phase into iron-rich and chromium-rich nanophases occurs, with no change in the mechanical properties of the austenite phase. This type of embrittlement is due to precipitation hardening, which makes the material become brittle and prone to cracking.












